Theacrine
| |

| |
| Names | |
|---|---|
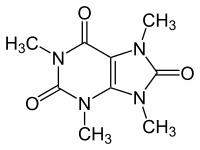
| Preferred IUPAC name
1,3,7,9-Tetramethyl-7,9-dihydro-1H-purine-2,6,8(3H)-trione | |
| Other names
1,3,7,9-Tetramethyluric acid; Temurin; Temorine; Tetramethyluric acid; Tetramethyl uric acid; TeaCrine (trade name)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.017.268 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H12N4O3 | |
| Molar mass | 224.220 g·mol−1 |
| Melting point | 226 °C (439 °F; 499 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Theacrine, also known as 1,3,7,9-tetramethyluric acid, is a purine alkaloid found in Cupuaçu (Theobroma grandiflorum) and in a Chinese tea known as kucha (Chinese: 苦茶; pinyin: kǔ chá; lit. 'bitter tea') (Camellia assamica var. Kucha).[1][2] It shows anti-inflammatory and analgesic effects and appears to affect adenosine signalling in a manner similar to caffeine.[2][3] In kucha leaves, theacrine is synthesized from caffeine in what is thought to be a three-step pathway.[2] Theacrine and caffeine are structurally similar.
Pharmacology
[edit]Pharmacodynamics
[edit]The exact mechanism of action of theacrine is uncertain, as no binding affinities have been published. However, animal research involving selective A1 and A2A adenosine agonists found theacrine pretreatment attenuated the expected motor depression induced by adenosine agonism, indicating that theacrine is likely an adenosine antagonist.[2]
Administration of selective dopamine D1 and D2 antagonists demonstrate that, similarly to caffeine,[4] theacrine's actions are in part mediated by dopamine receptors.[2] This should not be taken as evidence that theacrine directly interacts with and/or augments dopamine receptors distinct from caffeine, as some marketers have misleadingly claimed.
Pharmacokinetics
[edit]Theacrine has half-life of 30 to 33 hours.[5]
Safety
[edit]Theacrine has demonstrated clinical safety and non-habituating effects in healthy humans over eight weeks of daily use at up to 300 mg/day.[6] Moreover, there was no evidence of the tachyphylaxis typical of neuroactive agents like caffeine and other stimulants.[6]
In animal studies, theacrine has an LD50 of 810 mg/kg,[3][6] compared to 265 mg/kg for caffeine.[7]
See also
[edit]References
[edit]- ^ Zheng XQ, Ye CX, Kato M, Crozier A, Ashihara H (2002). "Theacrine (1,3,7,9-tetramethyluric acid) synthesis in leaves of a Chinese tea, kucha (Camellia assamica var. Kucha)". Phytochemistry. 60 (2): 129–34. Bibcode:2002PChem..60..129Z. doi:10.1016/s0031-9422(02)00086-9. PMID 12009315.
- ^ a b c d e Feduccia AA, Wang Y, Simms JA, Yi HY, Li R, Bjeldanes L, Ye C, Bartlett SE (2012). "Locomotor activation by theacrine, a purine alkaloid structurally similar to caffeine: Involvement of adenosine and dopamine receptors". Pharmacology Biochemistry and Behavior. 102 (2): 241–248. doi:10.1016/j.pbb.2012.04.014. PMID 22579816. S2CID 31549989.
- ^ a b Wang Y, Yang X, Zheng X, Li J, Ye C, Song X (2010). "Theacrine, a purine alkaloid with anti-inflammatory and analgesic activities". Fitoterapia. 81 (6): 627–631. doi:10.1016/j.fitote.2010.03.008. PMID 20227468.
- ^ Garrett BE, Holtzman SG (January 1994). "D1 and D2 dopamine receptor antagonists block caffeine-induced stimulation of locomotor activity in rats". Pharmacology, Biochemistry, and Behavior. 47 (1): 89–94. doi:10.1016/0091-3057(94)90115-5. ISSN 0091-3057. PMID 7906891. S2CID 23508010.
- ^ Mondal G, Wang YH, Butawan M, Bloomer RJ, Yates R (2021-01-06). Caffeine and Methylliberine: A Human Pharmacokinetic Interaction Study (Report). Pharmacology and Therapeutics. doi:10.1101/2021.01.05.21249234.
- ^ a b c Taylor L, Mumford P, Roberts M, Hayward S, Mullins J, Urbina S, Wilborn C (2016). "Safety of TeaCrine®, a non-habituating, naturally-occurring purine alkaloid over eight weeks of continuous use". Journal of the International Society of Sports Nutrition. 13: 2. doi:10.1186/s12970-016-0113-3. PMC 4711067. PMID 26766930.
- ^ Warszawski D, Gorodischer R, Kaplanski J (1978). "Comparative toxicity of caffeine and aminophylline (theophylline ethylenediamine) in young and adult rats". Biology of the Neonate. 34 (1–2): 68–71. doi:10.1159/000241107. PMID 698326.

