User:DiverDave/Deep sea communities
| This is not a Wikipedia article: This is a workpage, a collection of material and work in progress that may or may not be incorporated into an article. It should not necessarily be considered factual or authoritative. |
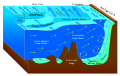
The deep sea or aphotic zone is found at a depth of 1000 meters or more. The aphotic zone in turn consists of three subzones: the bathyal, abyssal, and hadal zones. The deepest place in the deep sea is the Challenger Deep, located at the bottom of the Mariana Trench near Guam. At a depth of 10,911 meters (35,798 feet or 6.77 miles), the barometric pressure is about 11,318 metric tons-force per square meter (110.99 MPa). If Mount Everest were submerged there, its peak would be more than a mile beneath the surface.
Little or no sunlight penetrates to this depth, and most of the endemic organisms of the deep sea rely on falling organic matter produced in the photic zone above for subsistence. For this reason scientists assumed until recently that life would be sparse in the deep ocean. However, recent research has revealed that life is in fact abundant in the deep ocean.
The deep sea is an extremely hostile environment, characterized by barometric pressures between 20 - 1000 atmospheres, temperatures between -0.5°C - 4°C, and a relatively low oxygen concentration. Most fish that have evolved in this harsh environment are not capable of surviving under laboratory conditions, and attempts to study them alive in captivity have been unsuccessful thus far. For this reason little is known about them. As such, most species are known only to scientists and have therefore retained only their scientific names.
More is known about the moon than the deepest parts of the ocean.[1] Until the late 1970s little was known about the possibility of life on the deep ocean floor but the discovery of thriving colonies of shrimp and other organisms around hydrothermal vents changed that. Before the discovery of the undersea vents, all life was thought to be driven by the sun. But these organisms get their nutrients from the earth's mineral deposits directly. These organisms thrive in completely lightless and anaerobic environments, in highly saline water that may reach 300 °F (150 °C), drawing their sustainance from hydrogen sulfide, which is highly toxic to all terrestrial life. The revolutionary discovery that life can exist without oxygen or light significantly increases the chance of there being life elsewhere in the universe. Scientists now speculate that Europa, one of Jupiter's moons, may have conditions that could support life beneath its surface which is speculated to be a liquid ocean beneath the icy crust.
History
[edit]The Challenger Deep is the deepest surveyed point of all of Earth's oceans; it is located at the southern end of the Mariana Trench near the Mariana Islands group. The depression is named after HMS Challenger, whose researchers made the first recordings of its depth on 23 March 1875 at station 225. The reported depth was 4,475 fathoms (8184 meters) based on two separate soundings. In 1960, Don Walsh and Jacques Piccard descended to the bottom of the Challenger Deep in the Trieste bathyscaphe. At this great depth a small flounder-like fish was seen moving away from the spotlight of the bathyscaphe. Since then, no manned craft has ever returned to the Challenger Deep.
The Japanese remote operated vehicle (ROV) Kaiko became the second vessel to reach the bottom of the Challenger Deep in March 1995. Nereus, a hybrid remotely operated vehicle (HROV) of the Woods Hole Oceanographic Institution, is currently the only vehicle capable of exploring ocean depths beyond 7000 meters. Nereus reached a depth of 10,902 meters at the Challenger Deep on May 31 2009.[2][3] On 1 June 2009, sonar mapping of the Challenger Deep by the Simrad EM120 multibeam sonar bathymetry system aboard the R/V Kilo Moana indicated a maximum depth of 10971 meters (6.82 miles). The sonar system uses phase and amplitude bottom detection, with an accuracy of better than 0.2% of water depth (this is an error of about 22 meters at this depth).[3][4]
The first discovery of any deep-sea chemosynthetic community including higher animals was unexpectedly made at hydrothermal vents in the eastern Pacific Ocean during geological explorations (Corliss et al., 1979).[5] Two scientists, J. Corliss and J. van Andel, first witnessed dense chemosynthetic clam beds from the submersible DSV Alvin on February 17, 1977, after their unanticipated discovery using a remote camera sled two days before.[5]
Characteristics of the deep sea environment
[edit]Lack of light
[edit]
The ocean can be conceptualized as being divided into various zones, depending on depth, and presence or absence of sunlight. Nearly all life forms in the ocean depend on the photosynthetic activities of phytoplankton and other marine plants to convert carbon dioxide into organic carbon, which is the basic building block of organic matter. Photosynthesis in turn requires energy from sunlight to drive the chemical reactions that produce organic carbon.[6]
The stratum of the water column nearest the surface of the ocean (sea level) is referred to as the photic zone. The photic zone can be subdivided into two different vertical regions. The uppermost portion of the photic zone, where there is adequate light to support photosynthesis by phytoplankton and plants, is referred to as the euphotic zone (also referred to as the epipelagic zone, or surface zone).[7] The lower portion of the photic zone, where the light intensity is insufficient for photosynthesis, is called the disphotic zone (disphotic means "poorly lit" in Greek).[8] The disphotic zone is also referred to as the mesopelagic zone, or the twilight zone.[9] Its lowermost boundary is at a thermocline of 12 °C (54 °F), which, in the tropics generally lies between 200 and 1000 meters.[10]
The euphotic zone is somewhat arbitrarily defined as extending from the surface to the depth where the light intensity is approximately 0.1–1% of surface sunlight irradiance, depending on season, latitude and degree of water turbidity.[7][8] In the clearest ocean water, the euphotic zone may extend to a depth of about 150 meters,[7] or rarely, up to 200 meters.[9] Dissolved substances and solid particles absorb and scatter light, and in coastal regions the high concentration of these substances causes light to be attenuated rapidly with depth. In such areas the euphotic zone may be only a few tens of meters deep or less.[7][9] The disphotic zone, where light intensity is considerably less than 1% of surface irradiance, extends from the base of the euphotic zone to about 1000 meters.[10] Extending from the bottom of the photic zone down to the seabed is the aphotic zone, a region of perpetual darkness.[9][10]
Since the average depth of the ocean is about 4300 meters,[11] the photic zone represents only a tiny fraction of the ocean’s total volume. However, due to its capacity for photosynthesis, the photic zone has the greatest biodiversity and biomass of all oceanic zones. Nearly all primary production in the ocean occurs here. Any life forms present in the aphotic zone must either be capable of movement upwards through the water column into the photic zone for feeding, or must rely on material sinking from above,[12] or must find another source of energy and nutrition, such as occurs in chemosynthetic archaea found near hydrothermal vents and cold seeps.
Hyperbaricity
[edit]
These animals have evolved to survive the extreme pressure of the sub-photic zones. The pressure increases by about one atmosphere every ten meters. To cope with the pressure, many fish are rather small, usually not exceeding 25 cm in length. Also, scientists have discovered that the deeper these creatures live, the more gelatinous their flesh and more minimal their skeletal structure. These creatures have also eliminated all excess cavities that would collapse under the pressure, such as swim bladders[13].
Pressure is the greatest environmental factor acting on deep-sea organisms. Pressure increases 1 atmosphere (atm) for each 10 m in depth. In the deep sea, although most of the deep sea is under pressures between 200 and 600 atm, the range of pressure is from 20 to 1,000 atm. Pressure exhibits a great role in the distribution of deep sea organisms. Until recently, people lacked detailed information on the direct effects of pressure on most deep-sea organisms, because virtually all organisms trawled from the deep sea arrived at the surface dead or dying. With the advent of traps that incorporate a special pressure-maintaining chamber, undamaged larger metazoan animals have been retrieved from the deep sea in good condition. Some of these have been maintained for experimental purposes, and we are obtaining more knowledge of the biological effects of pressure.
Temperature
[edit]The two areas of greatest and most rapid temperature change in the oceans are the transition zone between the surface waters and the deep waters, the thermocline, and the transition between the deep-sea floor and the hot water flows at the hydrothermal vents. Thermoclines vary in thickness from a few hundred meters to nearly a thousand meters. Below the thermocline, the water mass of the deep ocean is cold and far more homogeneous. Thermoclines are strongest in the tropics, where the temperature of the epipelagic zone is usually above 20°C. From the base of the epipelagic, the temperature drops over several hundred meters to 5 or 6°C at 1,000 meters. It continues to decrease to the bottom, but the rate is much slower. Below 3,000 to 4,000 m, the water is isothermal.
At any given depth, the temperature is practically unvarying over long periods of time. There are no seasonal temperature changes, nor are there any annual changes. No other habitat on earth has such a constant temperature.
Hydrothermal vents are the direct contrast with constant temperature. In these systems, the temperature of the water as it emerges from the "black smoker" chimneys may be as high as 400°C (it is kept from boiling by the high hydrostatic pressure) while within a few meters it may be back down to 2 - 4°C.[14]
Salinity
[edit]
Salinity is remarkably constant throughout the depths of the deep sea. There are two notable exceptions to this rule:
- 1. In the Mediterranean Sea, water loss through evaporation greatly exceeds input from precipitation and river runoff. Because of this, salinity in the Mediterranean is higher than in the Atlantic Ocean. [15] Evaporation is especially high in its eastern half, causing the water level to decrease and salinity to increase in this area.[16] The resulting pressure gradient pushes relatively cool, low-salinity water from the Atlantic Ocean across the basin. This water warms and becomes saltier as it travels eastward, then sinks in the region of the Levant and circulates westward, to spill back into the Atlantic over the Strait of Gibraltar.[17] The net effect of this is that at the Strait of Gibraltar, there is an eastward surface current of cold water of lower salinity from the Atlantic, and a simultaneous westward current of warm saline water from the Mediterranean in the deeper zones. Once back in the Atlantic, this chemically distinct Mediterranean Intermediate Water can persist for thousands of kilometers away from its source.[18]
- 2. Brine pools are large areas of brine on the seabed. These pools are bodies of water that have a salinity that is three to five times greater than that of the surrounding ocean. For deep sea brine pools the source of the salt is the dissolution of large salt deposits through salt tectonics. The brine often contains high concentrations of methane, providing energy to chemosynthetic extremophiles that live in this specialized biome. Brine pools are also known to exist on the Antarctic continental shelf where the source of brine is salt excluded during formation of sea ice. Deep sea and Antarctic brine pools can be toxic to marine animals. Brine pools are sometimes called seafloor lakes because the dense brine creates a halocline which does not easily mix with overlying seawater. The high salinity raises the density of the brine, which creates a distinct surface and shoreline for the pool.[19]
Biological adaptations to the deep sea environment
[edit]Regions below the epipelagic are divided into further zones, beginning with the mesopelagic which spans from 200 to 1000 below sea level, where a little light penetrates while still being insufficient for primary production. Below this zone the deep sea proper begins, consisting of the aphotic bathypelagic, abyssopelagic and hadopelagic. Food consists of falling organic matter known as 'marine snow' and carcasses derived from the productive zone above, and is scarce both in terms of spatial and temporal distribution.
Instead of relying on gas for their buoyancy, many species have jelly-like flesh consisting mostly of glycosaminoglycans, which has very low density.[20] It is also common among deep water squid to combine the gelatinous tissue with a flotation chamber filled with a coelomic fluid made up of the metabolic waste product ammonium chloride, which is lighter than the surrounding water.
The midwater fish have special adaptations to cope with these conditions—they are small, usually being under 25 centimetres (10 in); they have slow metabolisms and unspecialized diets, preferring to sit and wait for food rather than waste energy searching for it. They have elongated bodies with weak, watery muscles and skeletal structures. They often have extendable, hinged jaws with recurved teeth. Because of the sparse distribution and lack of light, finding a partner with which to breed is difficult, and many organisms are hermaphroditic.
Because light is so scarce, fish often have larger than normal, tubular eyes with only rod cells. Their upward field of vision allows them to seek out the silhouette of possible prey. Prey fish however also have adaptations to cope with predation. These adaptations are mainly concerned with reduction of silhouette, a form of camouflage. The two main methods by which this is achieved are reduction in the area of their shadow by lateral compression of the body, and counter illumination via bioluminescence. This is achieved by production of light from ventral photophores, which tend to produce such light intensity to render the underside of the fish of similar appearance to the background light. For more sensitive vision in low light, some fish have a retroreflector behind the retina. Flashlight fish have this plus photophores, which combination they use to detect eyeshine in other fish (see Tapetum lucidum).
It is important to realize that organisms in the deep sea are almost entirely reliant upon sinking living and dead organic matter which falls at approximately 100 meters per day.[21] In addition to this, only about 1-3% of the production from the surface reaches the sea bed mostly in the form of marine snow - as mentioned above. Larger food falls, such as whale carcasses, also occur and studies have shown that these may happen more often than currently believed. There are lots of scavengers that feed primarily or entirely upon large food falls and the distance between whale carcasses is estimated to only be 8 kilometers.[22] In addition, there are a number of filter feeders that feed upon organic particles using tentacles, such as Freyella elegans.[23]
Marine bacteriophages play an important role in cycling nutrients in deep sea sediments. They are extremely abundant (between 5x1012 and 1x1013 phages per square meter) in sediments around the world. [24]
To survive, these creatures have much slower metabolisms and therefore can survive using little oxygen. They can also go months without food. Most food comes from either organic material that falls from above or from eating other creatures that have derived their food through the process of chemosynthesis. Because of the sparse distribution of creatures, there is always at least some oxygen and food. Also, instead of using energy to search for food, these creatures use particular adaptations to ambush prey.
Adaptations to darkness
[edit]
As the photic zone rarely extends for more than a few hundred meters below the water, bioluminescence is by far the most common source of available light at these depths. The chemical process of bioluminescence employs an enzyme called luciferase to catalyze the oxidation of luciferin (a biological pigment) to oxyluciferin, generating a photon in the process, as follows:
- luciferin + ATP → luciferyl adenylate + PPi
- luciferyl adenylate + O2 → oxyluciferin + AMP + light + CO2).[25]
This reaction is very energetically efficient; nearly all of the energy input into the reaction is transformed into light. Luciferin is regenerated by other enzymes, or replaced through dietary uptake.[26] In many cases, the luciferin exists within bacteria (such as Vibrio fischeri), which live within specialized tissues of certain squid and fish.


There are many types of luciferins, though they all share the use of reactive oxygen species to emit light.[27] Besides luciferins, organisms of the deep sea employ other types of bioluminescent proteins, including coelenterazine, an imidazopyrazinone found in certain chaetognatha, cnidaria, copepods, ctenophora, radiolarians, fish, squid, and shrimp. Coelenterazine is the prosthetic group in the protein aequorin, responsible for blue light emission.[28] Dinoflagellate luciferin is a chlorophyll derivative found in dinoflagellates, which are often responsible for the phenomenon of nighttime ocean phosphorescence. A very similar type of luciferin is found in some types of euphausiid shrimp. Vargulin is another bioluminescent protein found in certain ostracods, as well as the Poricthys genus of toadfishes. Like coelenterazine, it is an imidazopyrazinone and primarily emits blue light.
Many deep sea organisms employ bioluminescence to light their way or attract a mate. Some creatures, such as the anglerfish have a concentration of photophores in a small limb that protrudes from their bodies, which they use as a lure to catch curious fish. Bioluminescence can also confuse would-be predators.
Bioluminescent fish include:[29]
- Anglerfishes (Lophiiformes order)
- Cookiecutter shark (Isistius brasiliensis)
- Flashlight fishes (Anomalopidae family)
- Marine hatchetfishes (Sternoptychinae subfamily)
- Midshipman fishes (Poricthys genus, not a deep sea fish)
- Pineconefishes (Monocentridae family)
- Rattails (Macrouridae family)
- Gulper eels (Saccopharyngiformes, an order of ray-finned fishes including the Cyematidae, Eurypharyngidae, Monognathidae, and Saccopharyngidae)
- Viperfishes (Chauliodus genus)
Bioluminescent marine invertebrates include:
- many cnidarians
- certain Ctenophores or "comb jellies"
- certain echinoderms (e.g. Ophiurida)
- certain crustaceans
- certain chaetognaths
- certain molluscs
- certain clams, bivalves
- certain nudibranchs, sea slugs
- Octopus
- the order Teuthida
In addition to the capacity for bioluminescence, many fish have very large eyes with retinas constructed only of cone cells, which significantly increases their sensitivity to available light. These selective adaptations are helpful but not sufficient by themselves to overcome the obstacles posed by the blackness of the abyss. Ultimately, the lack of light requires organisms to rely on senses other than vision to find food, avoid predators, and find mates. For example, many animals have also developed large antennae to augment their peripheral vision. Many of these fish have evolved to be hermaphroditic, thus doubling the opportunity to reproduce. Many creatures have also developed very strong senses of smell to detect the pheromones released by potential mates. Lack of available light may also have a selective effect on methods of locomotion.
Deep-sea gigantism
[edit]
The term deep-sea gigantism describes an effect that living at such depths has on some creatures' sizes, especially relative to the size of relatives that live in different environments. These creatures are generally many times bigger than their smaller counterparts. The Giant Isopod (related to the common pill bug) exemplifies this. Scientists haven't been able to explain deep-sea gigantism, with the exception of the giant tube worm. Scientists believe these creatures are much larger than shallower-water tube worms because they live on hydrothermal vents that expel huge amounts of resources. They believe that, since the creatures don't have to expend energy regulating body temperature and have a smaller need for activity, they can allocate more resources to bodily processes.
There are also cases of deep-sea creatures being abnormally small, such as the lantern shark, which fits in an adult human's palm. [30]
Sources of energy and nutrients
[edit]Marine snow
[edit]Because of the sparsity of food, the organisms living on and in the bottom are generally opportunistic. They have special adaptations for this extreme environment: rapid growth, an effective larval dispersal mechanism and the ability to use transient food resources. One typical example is wood-boring bivalves, which feed on the organic matter from wood and other phytodetritus.
The upper photic zone (or euphotic zone) of the ocean is filled with particulate organic matter (POM), especially in the coastal and the upwelling areas. Most POM is small and light; it may take hundreds or even thousands of years for these particles to settle through the water column into the deep ocean. This time delay is long enough for the particles to be remineralized and taken up by organisms in the food web.
Scientists at Woods Hole Oceanographic Institution examined the rate of sinking three decades ago in the deep Sargasso Sea.[31] They found that the POM had been repackaged into much larger particles which sink at a much greater rate, falling like snow. They coined the term marine snow at that time.
Whale falls
[edit]For the deep-sea creatures, a dead whale is the most exciting event. A dead whale can bring hundreds of tons of organic matter to the bottom. Whale-fall community progresses through three stages:[32]
- Mobile scavenger stage: Big and mobile deep-sea animals arrive at the site almost immediately after whales fall on the bottom. Amphipods, crabs, sleeper sharks and hagfish are all scavengers.
- Opportunistic stage: One interesting genus is Osedax.[33] Osedax is an interesting tubeworm. The larva is born without sex. The surrounding environment determines the sex of the larva. When a larva settles on a whale bone, it turns into a female; when a larva settles on or in a female, it turns into a dwarf male. One female Osedax can carry more than 200 of these male individuals in its oviduct.
- Sulfophilic stage: Further decomposition of bones and seawater sulfate reduction happen at this stage. Bacteria create a sulphide-rich environment analogous to hydrothermal vents. Polynoids, bivalves, gastropods and other sulphur-loving creatures move in.
Chemosynthesis
[edit]Since at such deep levels, there is little to no sunlight, photosynthesis is impossible as a means of energy production, leaving some creatures with the quandary of how to produce food for themselves. For the giant tube worm, this answer comes in the form of bacteria that live inside of it. These bacteria are capable of chemosynthesis and live inside of the giant tube worm, which lives on hydrothermal vents. These vents spew very high amounts of chemicals that these bacteria can transform into energy. These bacteria can also grow freely of a host and create mats of bacteria on the sea floor around hydrothermal vents, where they serve as food to other creatures. Bacteria are a key energy source in the food chain. This source of energy creates large populations in areas around hydrothermal vents, which provides scientists an easy stop for research.[34]
There are a number of species that do not primarily rely upon dissolved organic matter for their food and these are found at hydrothermal vents. One example is the symbiotic relationship between the tube worm Riftia and chemosynthetic bacteria. It is this chemosynthesis that supports the complex communities that can be found around hydrothermal vents.[35] These complex communities are one of the only ecosystems on the planet that do not rely upon sunlight for the creation of energy.[36]

The term 'Deep Sea refers to organisms that live below the photic zone of the ocean. These creatures must survive in extremely harsh conditions; such as hundreds of atmospheres of pressure, small amounts of oxygen, very little food, no sunlight, and constant, extreme cold. Most creatures have to depend on food floating down from above.
These creatures live in very harsh environments such as the abyssal or hadal zones, which, being thousands of meters below the surface, are almost completely devoid of light. The water is very cold (between 3 and 10 degrees Celsius, or 37 and 50 degrees Fahrenheit), and has low oxygen levels. Due to the depth, the pressure is between 20 and 1,000 atmospheres. Creatures that live thousands of feet deep in the ocean have adapted to the high pressure, lack of light, and other factors.
Because of the high pressure, low temperature and absence of light, prior to the nineteenth Century scientists assumed life was sparse in the deep ocean. In the 1870s Sir Charles Thompson and colleagues aboard the Challenger expedition discovered many deep-sea creatures of widely varying types. This discovery raised another question: how can these creatures obtain food to feed themselves? In answering that question scientists have found three food sources supporting deep-sea creatures: marine snow, big organism falls, and chemosynthesis at hydrothermal vents.
Chemosynthetic communities
[edit]

Aside from whale fall communities, ecosystems found at cold seeps and hydrothermal vents are similar in that they are the only known communities that do not rely on photosynthesis for food and energy production. Seep vestimentiferans are usually thinner, have slower growth rates, and have greater longevity than their vent relatives [10]. For example, a 2-m-long Lamellibrachia luymesi individual is estimated to be more than 200 y old and hence represents the longest-lived animal on earth [11,12]. At seeps, geological processes causing fluid and gas seepage can last hundreds to millions of years, whereas hydrothermal vents often have a lifespan on the order of decades. Vent tubeworm colonies will die when their chimneys stop venting, i.e., delivering sulfide, so they are adapted to a rapidly changing environment, as typified by their fast growth and high reproduction.
Previously marine biologists assumed that vent organisms were dependent on a rain of detritus from the upper levels of the ocean, like deep sea organisms are. This would leave them dependent on plant life and thus the sun. Some hydrothermal vent organisms do consume this rain, but with only such a system, life forms would be very sparse. Compared to the surrounding sea floor, however, hydrothermal vent zones have a density of organisms 10,000 to 100,000 times greater. Hydrothermal vent communities are able to sustain such vast amounts of life because vent organisms depend on chemosynthetic bacteria for food. The water that comes out of the hydrothermal vent is rich in dissolved minerals and supports a large population of chemo-autotrophic bacteria. These bacteria use sulfur compounds, particularly hydrogen sulfide, a chemical highly toxic to most known organisms, to produce organic material through the process of chemosynthesis.
The first discovery of any deep-sea chemosynthetic community was unexpectedly made at hydrothermal vents in the eastern Pacific Ocean during geological explorations. Two scientists, J. Corliss and J. van Andel, observed dense chemosynthetic clam beds from the submersible DSV Alvin in February 1977. Similar communities were first discovered in the Eastern Gulf of Mexico in 1983 on another Alvin cruise investigating the bottom of the Florida Escarpment in areas of cold brine seepage where they unexpectedly discovered tubeworms and mussels. More chemosynthetic communities were discovered in the Central Gulf of Mexico between 1984 and 1986. These communities include tube worms, vesicomyid clams, mussels, and exposed carbonate outcrops with numerous gorgonian and Lophelia coral colonies.[5]
Four general community types have been described. These are communities dominated by:
- Vestimentiferan tube worms (Lamellibrachia c.f. barhami and Escarpia n.sp.)
- mytilid mussels (Seep Mytilid Ia, Ib, and III, and others)
- vesicomyid clams (Vesicomya cordata and Calyptogena ponderosa), and
- infaunal lucinid or thyasirid clams (Lucinoma sp. or Thyasira sp.).
These faunal groups tend to display distinctive characteristics in terms of how they aggregate, the size of aggregations, the geological and chemical properties of the habitats in which they occur and, to some degree, the heterotrophic fauna that occur with them. Many of the species found at these cold seep communities in the Gulf are new to science and remain undescribed. Individual lamellibranchid tube worms, the longer of two taxa found at seeps can reach lengths of 3 meters and live for hundreds of years. Average growth rate was about 2 cm/year for the Escarpia-like species and nearly 3 cm/year for lamellibrachids. These are slower growth rates than those of their hydrothermal vent relatives, but Lamellibrachia individuals can reach lengths 2-3 times that of the largest known hydrothermal vent species.[5] Individuals of Lamellibrachia sp. in excess of 3 meters have been collected on several occasions, representing probable ages in excess of 400 years.[37]
Spawning of female Lamellibrachia appears to result in the unique association of the large bivalve Acesta bullisi living permanently attached the anterior tube opening of the tubeworm feeding on the periodic egg release. Virtually all mature Acesta individuals are found on female rather than male tubeworms. Growth rates for methanotrophic mussels at cold seep sites have been found to be relatively high. Adult mussel growth rates were similar to those of mussels from a littoral environment at similar temperatures. Juvenile mussels at hydrocarbon seeps grow rapidly to reproductive size, but the growth rate drops markedly in adults. Both individuals and communities appear to be very long lived. These methane-dependent mussels have strict chemical requirements that tie them to areas of the most active seepage. Two associated species are always found associated with mussel beds – the gastropod Bathynerita naticoides and a small Alvinocarid shrimp.[5]
Heterotrophic species at seep sites are a mixture of species unique to seeps (particularly molluscs and crustacean invertebrates) and those that are a normal component from the surrounding environment. The diets of endemic seep-associated invertebrate consumers such as galatheid crabs and nerite gastropods are a mixture of seep and background production. At some sites, as much as 50 percent of their diets appears to be from the background.[5]
Extensive mats of free-living bacteria are also evident at all hydrocarbon seep sites visited to date. These bacteria may compete with the more complex fauna for sulfide and methane energy sources, but may also contribute substantially to overall production. The white mats were found to be an autotrophic sulfur bacteria Beggiatoa species, and the orange mats possessed an unidentified nonchemosynthetic metabolism.[5]
Seepage from hydrocarbon sources through faults towards the surface tends to be diffused through the overlying sediment, so hydrocarbon seep communities tend to be larger (a few hundred meters wide) than chemosynthetic communities found around the hydrothermal vents of the Eastern Pacific. Sites at which the seepage rate is very slow (~4 bbl/day) are only able to support simple microbial mats (Beggiatoa sp.). Sites at which the seepage rate is somewhat greater are able to support densely populated and diverse communities of chemosynthetic organisms (microbial mats, siboglinid tube worms, bathymodioline mussels, lucinid and vesycomyid clams, and associated organisms).[5]
Communities of chemosynthetic archaea and bacteria (such as those of the sulfide-oxidizing Beggiatoa genus), often arranged in large bacterial mats, are commonly found near cold seeps. These simple extremophile prokaryotes in turn support entire ecosystems consisting of more complex organisms, such as vesicomyid clams, siboglinid tube worms, soft corals, mytilid mussels, eelpouts, galatheid crabs, and alvinocarid shrimp. The deepest seep community discovered thus far is located in the Japan Trench, at a depth of 7700 meters.[38]
Taphonomic studies (death assemblages of shells) have revealed that some seep communities appear to have retained optimal habitat over geological time scales, and have probably existed in the Gulf of Mexico throughout the entire Pleistocene period. Powell reported evidence of mussel and clam communities persisting in the same sites for 500-4,000 years.[5]
Chemosynthetic bacteria and archaea are the primary producers, providing the energy and organic matter for the whole food web in hydrothermal vent and cold seep ecosystems. extremophiles convert the heat, methane, and sulfur compounds provided by black smokers into energy through a process called chemosynthesis. More complex life forms like clams and tubeworms feed on these organisms. The organisms at the base of the food chain also deposit minerals into the base of the black smoker, therefore completing the life cycle. Some theories indicate that life originated at hydrothermal vents from inorganic precursors.
Highly specialized deep sea ecosystems are common in the vicinity of hydrothermal vents and cold seeps. In this type of biome, the primary producers are typically chemotrophic archaea or bacteria. These extremophilic organisms obtain energy and nutrition from the oxidation of electron donors. Two broad categories of chemotrophs are the organotrophs, which obtain hydrogen or electrons from organic compounds, and the lithotrophs, which employ inorganic substrates for this purpose. In addition to deriving energy from chemical reactions, some of these organisms can synthesize all necessary organic compounds from carbon dioxide. Chemoautotrophs generally fall into several groups: methanogens, halophiles, and , and thermoacidophiles, and iron oxidizing bacteria. Hydrothermal vents also release large quantities of dissolved iron and sulfur into the deep ocean, allowing iron oxidizing, sulfur oxidizing and sulfur reducing bacteria to thrive. In addition, the high thermal gradient around vent systems means a wide variety of bacteria can coexist, each with its own specialized temperature niche. Regardless of the catalytic method used, chemoautotrophic bacteria provide a significant food source for deep sea ecosystems. These prokaryotes, both Archaea and Bacteria, process sulfides and methane through chemosynthesis into chemical energy. More complex organisms use this energy to power their own life processes. In exchange, the microbes are provided with both safety and a reliable source of food.
Unlike hydrothermal vents which are volatile and ephemeral environments, cold seeps emit hydrocarbons at a slow and predictable rate. Likely owing to the cooler temperatures and relative environmental stability, many cold seep organisms are much longer-lived than those inhabiting the areas around hydrothermal vents. For example the giant vestimentiferan tubeworm Lamellibrachia luymesi has an estimated lifespan of 170 to 250 years.[39] Lamellibrachia luymesi is entirely reliant on internal, sulfide-oxidizing bacterial symbionts for its nutrition.
Sulfate-reducing archaea of the genus Archaeoglobus produce energy by coupling the reduction of sulfate to sulfide with the oxidation of many different organic carbon sources, including complex polymers. Species of the Archaeoglobus genus are hyperthermophiles that can be found near hydrothermal vents, oil deposits, and hot springs. They grow at extremely high temperatures between 60 and 95 °C, with optimal growth at 83 °C.[40] Although the process of chemosynthesis is entirely microbial, chemosynthetic bacteria and their production can support thriving assemblages of higher organisms through symbiosis.[5]
The chemosynthetic bacteria grow into a thick mat which attracts other organisms such as amphipods and copepods which graze upon the bacteria directly. Larger organisms such as snails, shrimp, crabs, tube worms, fish, and octopuses form a food chain of predator and prey relationships above the primary consumers. The main families of organisms found around seafloor vents are annelids, pogonophorans, gastropods, and crustaceans, with large bivalves, vestimentiferan worms, and "eyeless" shrimp making up the bulk of non-microbial organisms.
New and unusual species are constantly being discovered in the neighborhood of black smokers – for instance, the Pompeii worm in the 1980s, and a scaly-foot gastropod in 2001 during an expedition to the Indian Ocean's Kairei hydrothermal vent field. The latter uses iron sulfides (pyrite and greigite) for the structure of its dermal sclerites (hardened body parts), instead of calcium carbonate. The extreme pressure of 2500 m of water (approximately 25 megapascals or 250 atmospheres) is thought to play a role in stabilizing iron sulfide for biological purposes. This armor plating probably serves as a defense against the venomous radula (teeth) of predatory snails in that community.
Over 300 new species have been discovered at hydrothermal vents,[41] many of them "sister species" to others found in geographically separated vent areas. It has been proposed that before the North American plate overrode the mid-ocean ridge, there was a single biogeographic vent region found in the eastern Pacific.[42] The subsequent barrier to travel began the evolutionary divergence of species in different locations. The examples of convergent evolution seen between distinct hydrothermal vents is seen as major support for the theory of natural selection and evolution as a whole.
Tube worms form an important part of the community around hydrothermal vents. The tube worms, like parasitic worms, absorb nutrients directly into their tissues. This is because tube worms have no mouth or even a digestive tract, so the bacteria live inside them. There are approximately 285 billion bacteria per ounce of tubeworm tissue. Tubeworms have red plumes which contain hemoglobin. Hemoglobin combines hydrogen sulfide and transfers it to the bacteria living inside the worm. In return the bacteria nourish the worm with carbon compounds. The two species that inhabit a hydrothermal vent are Tevnia jerichonana, and Riftia pachyptila. One community has been discovered dubbed 'Eel City', which consists predominantly of eels. Though eels are not uncommon, as mentioned earlier invertebrates typically dominate hydrothermal vents. Eel City is located near Nafanua volcanic cone, American Samoa.[43]
Other examples of the unique fauna who inhabit this ecosystem are scaly-foot gastropod Crysomallon squamiferum, a species of snail with a foot reinforced by scales made of iron and organic materials, and the Pompeii Worm Alvinella pompejana, which is capable of withstanding temperatures up to 80°C (176°F).
A species of phototrophic bacterium has been found living near a black smoker off the coast of Mexico at a depth of 2,500 m (8,200 ft). No sunlight penetrates that far into the waters. Instead, the bacteria, part of the Chlorobiaceae family, use the faint glow from the black smoker for photosynthesis. This is the first organism discovered in nature to exclusively use a light other than sunlight for photosynthesis.[44]
Nonchemosynthetic communities
[edit]Deep sea fish
[edit]
Deep sea fish is a term for fish that live below the photic zone of the ocean. The lanternfish is, by far, the most common deep sea fish. Other deep sea fish include the flashlight fish, cookiecutter shark, bristlemouths, anglerfish, and viperfish.
The fish of the deep sea are among the strangest and most elusive creatures on Earth. In this deep unknown lie many unusual creatures we still have yet to study. Since many of these fish live in regions where there is no natural illumination, they cannot rely solely on their eyesight for locating prey and mates and avoiding predators; deep sea fish have evolved appropriately to the extreme sub-photic region in which they live. Many deep sea fish are bioluminescent, with extremely large eyes adapted to the dark. Some have long feelers to help them locate prey or attract mates in the pitch black of the deep ocean. The deep sea angler fish in particular has a long fishing-rod-like adaptation protruding from its face, on the end of which is a bioluminescent piece of skin that wriggles like a worm to lure its prey. The lifecycle of deep sea fish can be exclusively deep water although some species are born in shallower water and sink on becoming adults.
Due to the poor level of photosynthetic light reaching deep sea environments, most fish need to rely on organic matter sinking from higher levels, or, in rare cases, hydrothermal vents for nutrients. This makes the deep sea much poorer in productivity than shallower regions. Consequently many species of deep sea fish are noticeably smaller and have larger mouths and guts than those living at shallower depths. It has also been found that the deeper a fish lives, the more jelly-like its flesh and the more minimal its bone structure. This makes them slower and less agile than surface fish.
Benthopelagic fish
[edit]
Benthopelagic fish inhabit the water just above the bottom, feeding on benthos and zooplankton.[48] Most dermersal fish are benthopelagic.[49]
Deep sea benthopelagic teleosts all have swimbladders. The dominate species, rattails and cusk eels, have considerable biomass. Other species include deep sea cods (morids), deep sea eels, halosaurs and notacanths.[50]
Benthopelagic sharks, like the deep sea squaloid sharks, achieve neutrally buoyancy with the use of large oil-filled livers.[51] Sharks adapt well to fairly high pressures. They can often be found on slopes down to about 2000 metres, scavenging on food falls such as dead whales. However, the energy demands of sharks are high, since they need to swim constantly and maintain a large amount of oil for buoyancy. These energy needs cannot be met in the extreme oligotrophic conditions that occur at great depths.[51]
Shallow water stingrays are benthic, and can lie on the bottom because of their negative buoyancy. Deep sea stingrays are benthopelagic, and like the squaloids have very large livers which give them neutral buoyancy.[51]
Benthopelagic fish can be divided into flabby or robust body types. Flabby benthopelagic fishes are like bathypelagic fishes; they have a reduced body mass, and low metabolic rates, expending minimal energy as they lie and wait to ambush prey.[52] An example of a flabby fish is the cusk-eel Acanthonus armatus,[53] a predator with a huge head and a body that is 90 percent water. This fish has the largest ears (otoliths) and the smallest brain in relation to its body size of all known vertebrates.[54]
Deepwater benthopelagic fish are robust, muscular swimmers that actively cruise the bottom searching for prey. They often live around features, such as seamounts, which have strong currents.[54] Commercial examples are the orange roughy and Patagonian toothfish.
Lanternfish
[edit]
Sampling via deep trawling indicates that lanternfish account for as much as 65% of all deep sea fish biomass.[55] Indeed, lanternfish are among the most widely distributed, populous, and diverse of all vertebrates, playing an important ecological role as prey for larger organisms. With an estimated global biomass of 550 - 660 million metric tonnes, several times the entire world fisheries catch, lanternfish also account for much of the biomass responsible for the deep scattering layer of the world's oceans. In the Southern Ocean, Myctophids provide an alternative food resource to krill for predators such as squid and the King Penguin. Although plentiful and prolific, currently only a few commercial lanternfish fisheries exist: These include limited operations off South Africa, in the sub-Antarctic, and in the Gulf of Oman.
Symphurus thermophilus
[edit]Symphurus thermophilus is a species of tonguefish, family Cynoglossidae, notable for being the only flatfish known to be an obligate inhabitant of hydrothermal vents. It is known from several widely dispersed locations in the western Pacific Ocean and occurs in great numbers. They are tolerant of harsh conditions and are often found in close association with elemental sulfur, including molten sulfur pools that exceed 180°C in temperature.[56] As they are not significantly different in appearance and feeding habits from other tonguefishes, they are thought to be relatively recent colonizers of vent ecosystems.[57]

S. thermophilus occurs only within relatively shallow active hydrothermal vent sites at a depth of 239-733 m, with most found between 300-400 m. Both adults and juveniles are found in the same habitats. Unusually for a vent fish, S. thermophilus favors environments that are rich in sulfur; they have been observed oriented vertically on solid sulfur walls, resting on beds of newly congealed sulfur adjacent to a rivulet of molten sulfur, and even on a thin crust of consolidated sulfur pebbles overlaying a molten sulfur bed with a temperature of 187°C (though the crust is considerably cooler).[57]
While many flatfish species prefer a fine substrate to burrow in, S. thermophilus frequent coarse substrates and are sometimes found over solid surfaces.[57] At the Kaikata Seamount, S. thermophilus was observed on coarse sand bottoms where water of 19-22°C was percolating through the sediment. At the Minami-Ensei Knoll, this species was found on white metachromatic sediments in water 5-10°C warmer than the ambient seawater. At the Kasuga-2 Seamount, it occurred on a variety of dark and light-colored gravel sediments and on bacterial mats.[58]
Where it occurs, S. thermophilus is often extremely abundant; it is the most numerous obligate vent vertebrate known to date. At the Kaikata Seamount, they are found in such numbers that the fish overlap one another on the bottom. Point densities at the Daikoku Seamount have been recorded as high as 392 individuals per square meter; these densities are an order of magnitude higher than flatfish densities reported anywhere else.[57]

The body is notably deep compared to other Symphurus species. The origin of the dorsal fin is located above the eyes and contains 88-94 rays. The dorsal fin pterygiophores and neural spines have a 1-2-2-2-2 interdigitation pattern. The pelvic fin is moderately long, contains 4 rays, and is connected to the body by a delicate membrane. The anal fin contains 74-80 fin rays. The caudal fin is relatively long and contains 14 rays. The scales are small and strongly ctenoid in shape, numbering 47-56 rows transversely and 100-112 rows longitudinally.[58]
The eyed side of the body is medium to dark chocolate brown in color, mottled with numerous dark, irregularly shaped blotches and white speckles. There are also five to eight darker, complete or incomplete crossbands. Some individuals have a white patch over two-thirds of the abdominal cavity, sometimes with bluish-green tints and bordered posteriorly by a black blotch. The abdominal area posterior to the gill opening is blackish brown and much darker than the rest of the body. Occasionally there are one or two irregular to nearly circular white spots along the body midline. The fin rays are dark at the base and lighter towards the tips, and there is an irregular dark spot at the base of the caudal fin. The blind side of the body is white, with scattered dark melanophores.[58]

S. thermophilus likely possesses extensive physiological and biochemical adaptations for coping with the harsh conditions around hydrothermal vents, such as temperature and pH fluctuations, and exposure to heavy metals. In particular, they must have high hemoglobin oxygen affinities and efficient respiratory systems to deal with the toxic hydrogen sulfide in venting fluid.[57] S. thermophilus is also capable of tolerating pH as low as 2, akin to sulfuric acid, and can rest over pools of molten sulfur without harm.[56] Individuals of S. thermophilus often show skeletal abnormalities such as undeveloped fin rays or fused bones, likely attributable to the vent environment.[58]
The diet of S. thermophilus varies significantly from seamount to seamount, with the only constant being polychaete worms, which are most important for individuals on Daikoku and Volcano-1 Seamounts. Other populations feed predominantly on crustaceans; the main prey item of S. thermophilus on the Nikko Seamount is the alvinocaridid shrimp Opaepele loihi, and on the Kasuga-2 Seamount they eat mostly palaemonid shrimp. The fish at these sites appear to be "sit and wait" predators, taking slow-moving shrimp that wander too close. By contrast, the fish at the Daikoku Seamount seem to be more active, opportunistic foragers; they do not eat many crustaceans and have been observed scavenging on dying fish that fall to the bottom after coming into contact with the volcanic plumes. Snails and sponge spicules have also been found in the stomachs of a few individuals, and in captivity they are known to consume any food offered to them. The large numbers of S. thermophilus found on sulfur crusts where there are no obvious prey items may feed directly on filaments of chemosynthetic bacteria. If so, this would represent a hitherto unknown behavior for vent fish species.[57][59] Compared to other flatfish, S. symphurus is slow-growing and long-lived, with a lifespan upwards of 10 years.[57]
Endangered species
[edit]A 2006 study by Canadian scientists has found five species of deep sea fish – roundnose grenadier, onion-eye grenadier, blue hake, spiny eel and spinytail skate – to be on the verge of extinction due to the shift of commercial fishing from continental shelves to the slopes of the continental shelves, down to depths of 1600 meters. The slow reproduction of these fish – they reach sexual maturity at about the same age as human beings – is one of the main reasons that they cannot recover from the excessive fishing.[60]
Deep water demersal fish occupy the benthic regions beyond the continental margins. On the continental slope, demersal fishes are common. They are more diverse than coastal demersal fish, since there is more habitat diversity. Further out are the abyssal plains. These flat, featureless regions occupy about 40 percent of the ocean floor. They are covered with sediment but largely devoid of benthic life (benthos). Deep sea benthic fishes are more likely to associate with canyons or rock outcroppings among the plains, where invertebrate communities are established. Undersea mountains (seamounts) can intercept deep sea currents, and cause productive upwellings which support benthic fish. Undersea mountain ranges can separate underwater regions into different ecosystems.[61] Rattails and brotulas are common, and other well established families are eels, eelpouts, hagfishes, greeneyes, batfishes and lumpfishes.[61]
The bodies of deep water demersal fishes are muscular with well developed organs. In this way they are closer to mesopelagic fishes than bathypelagic fishes. In other ways, they are more variable. Photophores are usually absent, eyes and swimbladders range from absent to well developed. They vary in size, and larger species, greater than one metre, are not uncommon.

Deep sea demersal fish are usually long and narrow. Many are eels or shaped like eels. This may be because long bodies have long lateral lines. Lateral lines detect low-frequency sounds, and some demersal fishes have muscles that drum such sounds to attract mates.[62] Smell is also important, as indicated by the rapidity with which demersal fish find traps baited with bait fish.
The main diet of deep sea demersal fish is invertebrates of the deep sea benthos and carrion. Smell, touch and lateral line sensitivities seem to be the main sensory devices for locating these.[63]
Like coastal demersal fish, deep sea demersal fish can be divided into benthic fish and benthopelagic fish, where the benthic fish are negatively buoyant and benthopelagic fish are neutrally buoyant.[63]
The availability of plankton for food diminishes rapidly with depth. At 1000 metres, the biomass of plankton is typically about 1 percent of that at the surface, and at 5000 metres about 0.01 percent.[50] Given there is no sunlight, energy enters deep water zones as organic matter. There are three main ways this happens. Firstly, organic matter can move into the zone from the continental landmass, for example, through currents that carry the matter down rivers, then plume along the continental shelf and finally spill down the continental slope. Other matter enters as particulate matter raining down from the overhead water column in the form of marine snow, or as sinking overhead plant material such as eelgrass, or as "large particles" such as dead fish and whales sinking to the bottom. A third way energy can arrive is through fish, such as vertically migrating mesopelagic fishes that can enter into the demersal zone as they ascend or descend. The demersal fish and invertebrates consume organic matter that does arrive, break it down and recycle it. A consequence of these energy delivery mechanisms is that the abundance of demersal fish and invertebrates gradually decrease as the distance from continental shorelines increases.[64]
Although deep water demersal fish species are not generally picky about what they eat, there is still some degree of specialisation. For example, different fish have different mouth sizes, which determines the size of the prey they can handle. Some feed mostly on benthopelagic organisms. Others fed mostly on epifauna (invertebrates on top of the seafloor surface, also called epibenthos), or alternatively on infauna (invertebrates that burrow into the seafloor substrate). Infauna feeders can have considerable sediment in their stomachs. Scavengers, such as snubnosed eels and hagfish, also eat infauna as a secondary food source.[65]
Some feed on carrion. Cameras show that when a dead fish is placed on the bottom, vertebrate and invertebrate scavengers appear very quickly. If the fish is large, some scavengers burrow in and eat it from the inside out. Some fish, such as grenadiers, also appear and start feeding on the scavenging inverebrates and amphipods. Other specialization is based on depth distribution. Some of the more abundant upper continental slope fish species, such as cutthroat eel and longfinned hake,[67] mainly feed on on epipelagic fish. But generally, the most abundant deep water demersal fish species feed on invertebrates.[65][68]
At great depths, food scarcity and extreme pressure limits the ability of fish to survive. The deepest point of the ocean is about 11,000 metres. Bathypelagic fishes are not normally found below 3,000 metres.[69] It may be that extreme pressures interfere with essential enzyme functions.[70]
The deepest-living fish known, the strictly benthic Abyssobrotula galatheae, eel-like and blind, feeds on benthic invertebrates. A living example was trawled from the bottom of the Puerto Rico Trench in 1970 from a depth of 8,370 metres (27,453 ft).[71][72]
In 2008, a shoal of 17 hadal snailfish, a species of deep water snailfish, was filmed by a UK-Japan team using remote operated landers at depths of 7.7 km (4.8 miles) in the Japan Trench in the Pacific. The fish were 30 centimetres long (12 in), and were darting about, using vibration sensors on their nose to catch shrimps. The team also reported that the appearance of the fish, unlike that of most deep sea fish, was surprisingly "cute", and that they were surprised by how active the fish were at these depths.[73][74]
Exponential decline of the deep sea ecosystem
[edit]Summary Background: Recent investigations suggest that biodiversity loss might impair the functioning and sustainability of ecosystems. Although deep-sea ecosystems are the most extensive on Earth, represent the largest reservoir of biomass, and host a large proportion of undiscovered biodiversity, the data needed to evaluate the consequences of biodiversity loss on the ocean floor are completely lacking. Results: Here, we present a global-scale study based on 116 deep-sea sites that relates benthic biodiversity to several independent indicators of ecosystem functioning and efficiency. We show that deep-sea ecosystem functioning is exponentially related to deep-sea biodiversity and that ecosystem efficiency is also exponentially linked to functional biodiversity. These results suggest that a higher biodiversity supports higher rates of ecosystem processes and an increased efficiency with which these processes are performed. The exponential relationships presented here, being consistent across a wide range of deep-sea ecosystems, suggest that mutually positive functional interactions (ecological facilitation) can be common in the largest biome of our biosphere. Conclusions: Our results suggest that a biodiversity loss in deep-sea ecosystems might be associated with exponential reductions of their functions. Because the deep sea plays a key role in ecological and biogeochemical processes at a global scale, this study provides scientific evidence that the conservation of deep-sea biodiversity is a priority for a sustainable functioning of the worlds’ oceans.
Introduction The accelerating loss of biological diversity poses serious concerns, exemplified by recent predictions that species loss might impair the functioning and the sustainability of terrestrial ecosystems [1–3]. The global scale of the biodiversity crisis has stimulated investigations that explore the relationships between biodiversity (expressed as the number, identity, and relative abundance of species), productivity, stability, and services in different ecosystems of the world [1–5]. Deep-sea sediments cover65%of the world’s surface. The microbial processes occurring there provide essential services, driving the nutrient regeneration and global biogeochemical cycles that are essential to sustain primary and secondary production in the oceans [6]. Deep-sea habitats are also the largest reservoirs of biomass and nonrenewable resources (e.g., gas hydrates and minerals) [6], and although the census of deep-sea life is in its infancy, there is increasing evidence that they host a large proportion of undiscovered biodiversity on our planet (from 0.3 to 8.33106 species) [5, 6]. Understanding the relationships between biodiversity and deep-sea ecosystem functioning is therefore crucial for understanding the functioning of our biosphere. Benthic faunal diversity provides an ideal tool for exploring the relationships between biodiversity and ecosystem functioning [7], and among benthic faunal taxa, nematodes are ideal model organisms. Nematodes are, indeed, the most abundant metazoans on Earth; in terrestrial ecosystems, they account for 80% of the abundance of multicellular animals, and in the deep sea, this proportion rises to more than 90% [8]. This phylum is also characterized by (1) very high species richness (i.e., among the most diverse of marine Phyla), (2) distinct and easily recognizable feeding types, and (3) life strategies that make it possible to also identify functional diversity traits [9]. Moreover, although comparative studies are rare, deep-sea nematode diversity appears to be related to that of other benthic components, including Foraminifera [10], macrofauna [11], and the richness of higher meiofaunal taxa (a group which includes 22 of the 35 modern animal Phyla; Ecosystem functioning involves several processes, which can be summarized as production, consumption and transfer of organic matter to higher trophic levels, organic matter decomposition, and nutrient regeneration. Terrestrial ecologists have related biodiversity to ecosystem functioning through analyses of ecosystem processes estimated by measuring the rates of energy and material flow between biotic and abiotic compartments (e.g., biomass production, organic matter decomposition, nutrient regeneration, or other measures of material production, transport, or loss) [2]. Applying the same approach through a series of independent and synoptic measures, we investigated the relationships between deep-sea biodiversity and ecosystem functioning. Deep-sea ecosystems lack photosynthetic primary production, and their functioning reflects the collective activities of animals, protists, and prokaryotes in exploiting and recycling the inputs of material from the photic zone. We therefore identified the following key processes: (1) benthic prokaryote production, (2) total meiofaunal biomass (a measure of the production of renewable resources by ecosystems), and (3) the rates at which organic matter is decomposed and recycled. The three independent indicators of ecosystem functioning represent key variables of deep-sea ecosystems as they regulate (1) the transfer of mobilized organic matter to higher trophic levels, (2) the ability of the ecosystem to transfer energy and material to higher trophic levels, thus providing indications of the heterotrophic production of the ecosystem, and (3) nutrient regeneration processes, which reflect the ability of ecosystems to sustain their functions over time. [75]
Deep sea research
[edit]
Humans have explored less than 2% of the ocean floor, and dozens of new species of deep sea creatures are discovered with every dive. The submarine DSV Alvin—owned by the US Navy and operated by the Woods Hole Oceanographic Institution (WHOI) in Woods Hole, Massachusetts—exemplifies the type of craft used to explore deep water. This 16 ton submarine can withstand extreme pressure and is easily maneuverable despite its weight and size.
However, studying deep sea creatures is problematic, since with the extreme change in pressure, and environment in general, these creatures can't survive for very long, if at all, on the surface. This makes in depth research difficult because so much of what we want to know about only occurs while the creature is alive. Recent developments have allowed scientists to look at these creatures more closely, and for a longer time. A marine biologist, Jeffery Drazen, has explored a solution, a pressurized fish trap. This captures a deep-water creature, and adjusts its internal pressure slowly to surface level as the creature is brought to the surface, in hopes that the creature can adjust. [76]
The deep sea is an environment totally inhospitable to humankind, and it should come as no surprise that it represents one of the least explored areas on Earth. Pressures even in the mesopelagic become too great for traditional exploration methods, demanding alternative approaches for deep sea research. Baited camera stations, small manned submersibles and ROVs (remotely operated vehicles) are three methods utilized to explore the ocean's depths. Because of the difficulty and cost of exploring this zone, current knowledge is limited. Pressure increases at approximately one atmosphere for every 10 meters meaning that some areas of the deep sea can reach pressures of above 1,000 atmospheres. This not only makes great depths very difficult to reach without mechanical aids, but also provides a significant difficulty when attempting to study any organisms that may live in these areas as their cell chemistry will be adapted to such vast pressures. If any fish or organisms from this depth were brought to the surface to be studied under laboratory conditions, the low atmospheric pressure would cause them to expand or even explode.
See also
[edit]- Bioluminescence
- Census of Marine Life
- Deep sea
- Deep sea fish
- Demersal fish
- Pelagic fish
- Submarine landslide
- The Blue Planet
- Deep Sea Foraminifera – Deep Sea Foraminifera from 4400m depth, Antarctica - an image gallery and description of hundreds of specimens
References
[edit]- ^ Cite error: The named reference
flannerywas invoked but never defined (see the help page). - ^ "Robot sub reaches deepest ocean". BBC News. 3 June 2009. Retrieved 2009-06-03.
- ^ a b University of Hawaii Marine Center (4 June 2009). "Daily Reports for R/V KILO MOANA June & July 2009". Honolulu, Hawaii: University of Hawaii. Retrieved 24 June 2010.
- ^ University of Hawaii Marine Center (4 June 2009). "Inventory of Scientific Equipment aboard the R/V KILO MOANA". Honolulu, Hawaii: University of Hawaii. Retrieved 18 June 2010.
- ^ a b c d e f g h i j Minerals Management Service, Gulf of Mexico OCS Region (Novemeber 2006). "Chapters 3: DESCRIPTION OF THE AFFECTED ENVIRONMENT". In Chris C. Oynes (ed.). Gulf of Mexico OCS Oil and Gas Lease Sales: 2007-2012. Western Planning Area Sales 204, 207, 210, 215, and 218. Central Planning Area Sales 205, 206, 208, 213, 216, and 222. Draft Environmental Impact Statement. Volume I (PDF). New Orleans: United States Department of the Interior, Minerals Management Service, Gulf of Mexico OCS Region. pp. 3-27–3-31. Retrieved 20 June 2010.
{{cite book}}: Check date values in:|date=(help) - ^ K.L. Smith, Jr, H.A. Ruhl, B.J. Bett, D.S.M. Billett, R.S. Lampitt & R.S. Kaufmann (17 November 2009). "Climate, carbon cycling, and deep-ocean ecosystems". PNAS. 106 (46): 19211–19218. doi:10.1073/pnas.0908322106. PMC 2780780. PMID 19901326.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Jorge Csirke (1997). "II. The Limits of Marine Productivity". In Edward A. Laws (ed.). El Niño and the Peruvian Anchovy Fishery (series: Global Change Instruction Program) (PDF). Vol. 9. Sausalito: University Science Books. pp. 118–121. doi:10.1023/A:1008801515441. ISBN 0935702806. Retrieved 18 June 2010.
{{cite book}}:|journal=ignored (help) - ^ a b Encyclopædia Britannica (2010). "Photic zone". Encyclopædia Britannica Online. Retrieved 18 June 2010.
{{cite web}}: External link in|publisher= - ^ a b c d Jeananda Col (2004). "Twilight Ocean (Disphotic) Zone". EnchantedLearning.com. Retrieved 18 June 2010.
{{cite web}}: External link in|publisher= - ^ a b c Ken O. Buesseler, Carl H. Lamborg, Philip W. Boyd, Phoebe J. Lam; et al. (27 April 2007). "Revisiting Carbon Flux Through the Ocean's Twilight Zone". Science. 316 (5824): 567–570. doi:10.1126/science.1137959. PMID 17463282. Retrieved 18 June 2010.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ National Oceanic and Atmospheric Administration (02 December 2008). "How deep is the ocean?". Washington, DC: National Oceanic and Atmospheric Administration. Retrieved 19 June 2010.
{{cite web}}: Check date values in:|date=(help) - ^ Cite error: The named reference
Smith2008was invoked but never defined (see the help page). - ^ The Deep Sea at MarineBio.org - Ocean biology, Marine life, Sea creatures, Marine conservation
- ^ Nybakken, James W. Marine Biology: An Ecological Approach. Fifth Edition. Benjamin Cummings, 2001. p. 136 - 141.
- ^ Paul R. Pinet (1996), Invitation to Oceanography (3rd ed.), St Paul, MN: West Publishing Co., p. 202, ISBN 0314063390
- ^ Pinet 1996, p. 206.
- ^ Pinet 1996, pp. 206–207.
- ^ Pinet 1996, p. 207.
- ^ NOAA exploration of a brine pool
- ^ http://www.astrobio.net/news/print.php?sid=617
- ^ [1]
- ^ R. N. Gibson, Harold (CON) Barnes, R. J. A. Atkinson, Oceanography and Marine Biology, An Annual Review. 2007. Volume 41: An Annual Review: Volume 41. Published by CRC Press, 2004 ISBN 0-415-25463-9, 9780415254632
- ^ http://www.nhm.ac.uk/nature-online/earth/oceans/deep-ocean/session3/index.html
- ^ Danovaro, Roberto; Dell'Anno, Antonio; Corinaldesi, Cinzia; Magagnini, Mirko; Noble, Rachel; Tamburini, Christian; Weinbauer, Markus (2008-08-28). "Major viral impact on the functioning of benthic deep-sea ecosystems". Nature. 454 (7208): 1084–1087. doi:10.1038/nature07268. PMID 18756250. Retrieved 2009-05-03.
- ^ "EC 1.13.12.5". IUBMB Enzyme Nomenclature. Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). Retrieved 21 June 2010.
- ^ BL Web: Chemistry
- ^ Hastings JW (1983). "Biological diversity, chemical mechanisms, and the evolutionary origins of bioluminescent systems". J. Mol. Evol. 19 (5): 309–21. doi:10.1007/BF02101634. PMID 6358519.
- ^ Shimomura O, Johnson FH (April 1975). "Chemical nature of bioluminescence systems in coelenterates". Proc. Natl. Acad. Sci. U.S.A. 72 (4): 1546–9. doi:10.1073/pnas.72.4.1546. PMC 432574. PMID 236561.
{{cite journal}}: CS1 maint: date and year (link) - ^ Monterey Bay Aquarium: Online Field Guide
- ^ Video: 12ft Crabs, Walking Fish and Mini Sharks: Deep Sea Creatures - Science - WeShow (US Edition)
- ^ "Marine Snow and Fecal Pellets".
- ^ Shana Goffredi, Unusual benthic fauna associated with a whale fall in Monterey Canyon, California, Deep-sea Research, 1295-1304, 2004
- ^ Noah K. Whiteman, Between a whale bone and the deep blue sea: the provenance of dwarf males in whale bone-eating tubeworms, Molecular Ecology, 4395–4397, 2008
- ^ Chemosynthesis
- ^ HW Jannasch. 1985. The Chemosynthetic Support of Life and the Microbial Diversity at Deep-Sea Hydrothermal Vents. Proceedings of the Royal Society of London. Series B, Biological Sciences, Vol. 225, No. 1240 (Sep. 23, 1985), pp. 277-297
- ^ HW Jannasch. 1985. The Chemosynthetic Support of Life and the Microbial Diversity at Deep-Sea Hydrothermal Vents. Proceedings of the Royal Society of London. Series B, Biological Sciences, Vol. 225, No. 1240 (Sep. 23, 1985), pp. 277-297
- ^ Fisher, C.R. (1995). "Characterization of habitats and determination of growth rate and approximate ages of the chemosynthetic symbiont-containing fauna". In MacDonald, I.R., W.W. Schroeder, and J.M. Brooks (ed.). Chemosynthetic ecosystems study: Final report. Volume 2: Technical report. New Orleans, LA: United States Department of the Interior, Minerals Management Service, Gulf of Mexico OCS Region. pp. 5.1–5.47.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ Cite error: The named reference
Morelle2008was invoked but never defined (see the help page). - ^ Antje Boetius (15 March 2005). "Microfauna–Macrofauna Interaction in the Seafloor: Lessons from the Tubeworm". PLOS Biology. 3 (3): e102. doi:10.1371/journal.pbio.0030102. PMC 1065708. PMID 15760275.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Hans-Peter Klenk, Rebecca A. Clayton, Jean-Francois Tomb, Owen White, Karen E. Nelson, Karen A. Ketchum, Robert J. Dodson, Michelle Gwinn, Erin K. Hickey; et al. (27 November 1997). "The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus". Nature. 390 (6658): 364–370. doi:10.1038/37052. PMID 9389475. Retrieved 20 June 2010.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Botos, Sonia. "Life on a hydrothermal vent". botos.com
- ^ Van Dover, Cindy Lee. "Hot Topics: Biogeography of deep-sea hydrothermal vent faunas". www.divediscover.whoi.edu
- ^ Astrobiology Magazine: Extremes of Eel City Retrieved 30 August 2007
- ^ Beatty, et al., 2005
- ^ Compagno, Leonard J.V. (1984). Sharks of the World: An Annotated and Illustrated Catalogue of Shark Species Known to Date. Rome: Food and Agricultural Organization. ISBN 9251013845.
- ^ Martin, R.A. Procellariidae and Pseudotriakidae: Finback & False Catsharks. ReefQuest Centre for Shark Research. Retrieved on February 14, 2009.
- ^ Froese, Rainer; Pauly, Daniel (eds.). "Pseudotriakis microdon". FishBase. August 2009 version.
- ^ Mauchline J and Gordon JDM (1986) "Foraging strategies of deep-sea fish"] Mar. Ecol. Prog. Ser. 27: 227-238. Download
- ^ Cite error: The named reference
seafloorwas invoked but never defined (see the help page). - ^ a b Bone 2008, p. 43.
- ^ a b c Bone 2008, p. 42.
- ^ Koslow JA (1996) "Energetic and life-history patterns of deep-sea benthic, benthopelagic and seamount-associated fish" Journal of Fish Biology, 49(sA) 54-74.
- ^ Froese, Rainer; Pauly, Daniel (eds.). "Acanthonus armatus". FishBase. August 2009 version.
- ^ a b Fine ML, Horn MH and Cox B (1987) "Acanthonus armatus, a Deep-Sea Teleost Fish with a Minute Brain and Large Ears" Proceedings of the Royal Society B, 230(1259)257-265.
- ^ Hulley, P. Alexander (1998). Paxton, J.R. & Eschmeyer, W.N. (ed.). Encyclopedia of Fishes. San Diego: Academic Press. pp. 127–128. ISBN 0-12-547665-5.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ a b Amos, J. (Dec 14, 2006). "Fish dance on sulphur cauldrons". BBC News. Retrieved on December 20, 2008.
- ^ a b c d e f g Cite error: The named reference
tylerwas invoked but never defined (see the help page). - ^ a b c d Munroe, T.A. and Hashimoto, J. (2008). "A new Western Pacific Tonguefish (Pleuronectiformes: Cynoglossidae): The first Pleuronectiform discovered at active Hydrothermal Vents". Zootaxa. 1839: 43–59. doi:10.11646/zootaxa.1839.1.2.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cite error: The named reference
dowerwas invoked but never defined (see the help page). - ^ Jennifer A. Devine, Krista D. Baker and Richard L. Haedrich; "Fisheries: Deep-sea fishes qualify as endangered" in Nature, vol 439, p. 29
- ^ a b Moyle and Cech, 2004, p. 587
- ^ Haedrich RL (1996) "Deep-water fishes: evolution and adaptation in the earth's largest living spaces" Journal of Fish Biology 49(sA):40-53.
- ^ a b Moyle and Cech, 2004, p. 588
- ^ Moyle and Cech, 2004, p. 594
- ^ a b Sedberry GR and Musick JA (1978) "Feeding strategies of some demersal fishes of the continental slope and rise off the mid-Atlantic coast of the USA" Marine Biology, 44:357-375.
- ^ Froese, Rainer; Pauly, Daniel (eds.). "Urophycis tenuis". FishBase. August 2009 version.
- ^ Froese, Rainer; Pauly, Daniel (eds.). "Phycis chesteri". FishBase. August 2009 version.
- ^ Moyle and Cech, 2004, p. 595
- ^ Nielsen, J.G. (1977). "The deepest living fish Abyssobrotula galatheae: a new genus and species of oviparous ophidioids (Pisces, Brotulidae)". Galathea Report. 14: 41–48.
- ^ Ryan P "Deep-sea creatures: The bathypelagic zone" Te Ara - the Encyclopedia of New Zealand. Updated 21 September 2007.
- ^ Nielsen JG (1977) "The deepest living fish Abyssobrotula galatheae: a new genus and species of oviparous ophidioids (Pisces, Brotulidae)". Galathea Report, 14: 41–48.
- ^ Froese, Rainer; Pauly, Daniel (eds.). "Abyssobrotula galatheae". FishBase. August 2009 version.
- ^ 'Deepest ever' living fish filmed BBC News, 7 October 2008.
- ^ Froese, Rainer; Pauly, Daniel (eds.). "Pseudoliparis amblystomopsis". FishBase. March 2009 version.
- ^ Roberto Danovaro, Cristina Gambi, Antonio Dell'Anno, Cinzia Corinaldesi, Simonetta Fraschetti, Ann Vanreusel, Magda Vincx and Andrew J. Gooday (08 January 2008). "Exponential Decline of Deep-Sea Ecosystem Functioning Linked to Benthic Biodiversity Loss" (PDF). Current Biology. 18 (1): 1–8. doi:10.1016/j.cub.2007.11.056. PMID 18164201. Retrieved 20 June 2010.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: multiple names: authors list (link) - ^ New Trap May Take Deep-Sea Fish Safely Out of the Dark
Further reading
[edit]External links
[edit]- Sea and Sky: Monsters of the Deep Sea - Details about specific creatures
- Marine Biology: The Deep Sea - General resource on deep sea creatures
- Monterey Bay Aquarium: Online Field Guide - Details about specific creatures
- The Bioluminescence Web Page - Good resource on bioluminescence
- NeMO Explorer - Good resource on chemosynthesis
- Deep Sea Monsters - Good resource on different kinds of deep sea fishes.
- http://www.pbs.org/wgbh/nova/abyss/life/bestiary.html