User:Hook0601
| Fish Temporal range: Middle Cambrian – Recent
| |
|---|---|

| |
| Diversity of various fish including sharks, stingrays, bony fish, jawless fish, and coelacanths. | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Olfactores |
| Subphylum: | Vertebrata |
| Groups included | |
| Cladistically included but traditionally excluded taxa | |
| This user is a participant in WikiProject Google. |
A fish (pl.: fish or fishes) is an aquatic, gill-bearing animal with a hard skull that lacks limbs with digits. This includes hagfish, lampreys, and both cartilaginous and bony fish. Approximately 95% of living fish species are ray-finned bony fish; around 99% of those are teleosts. As a group, if tetrapods are excluded, fish are paraphyletic and so do not form a taxonomic group.
The earliest organisms that can be classified as fish were soft-bodied chordates that first appeared during the Cambrian period. Although they lacked a true spine, they possessed notochords which allowed them to be more agile than their invertebrate counterparts. Fish would continue to evolve through the Paleozoic era, diversifying into a wide variety of forms. Many fish of the Paleozoic developed external armor that protected them from predators. The first fish with jaws appeared in the Silurian period, after which many (such as sharks) became formidable marine predators rather than just the prey of arthropods.
Most fish are ectothermic ("cold-blooded"), allowing their body temperatures to vary as ambient temperatures change, though some of the large active swimmers like white shark and tuna can hold a higher core temperature. Fish can acoustically communicate with each other, most often in the context of feeding, aggression or courtship.
Fish are an important resource for humans worldwide, especially as food. Commercial and subsistence fishers hunt fish in wild fisheries or farm them in ponds or in cages in the ocean. They are also caught by recreational fishers, kept as pets, raised by fishkeepers, and exhibited in public aquaria. Fish have had a role in culture through the ages, serving as deities, religious symbols, and as the subjects of art, books and movies.
Etymology
The word fish is inherited from Proto-Germanic, and is related to the Latin piscis and Old Irish īasc, though the exact root is unknown; some authorities reconstruct a Proto-Indo-European root *peysk-, attested only in Italic, Celtic, and Germanic.[1][2][3][4]
Evolution
Fossil history

The evolution of fish began about 530 million years ago during the Cambrian explosion. At this time the early chordates developed their skull and vertebral column.[5] The first fish lineages belong to the Agnatha, or jawless fish, such as Haikouichthys.[6] During the late Cambrian, other jawless forms such as conodonts first appeared.[5]
Jawed vertebrates appear in the fossil record from the Silurian, with giant armoured placoderms such as Dunkleosteus,[7] and the Acanthodii or spiny sharks. The jawed fish still extant also appeared during the late Silurian: the cartilaginous Chondrichthyes and the bony Osteichthyes. The bony fish evolved into two separate groups: the ray-finned Actinopterygii and the fleshy-finned Sarcopterygii.
During the Devonian, fish diversity greatly increased, especially among the ostracoderms and placoderms, and among the lobe-finned fish and early sharks, earning the Devonian the name "age of fishes".
Phylogeny
Fishes are a paraphyletic group, since any clade containing all fish, such as the Gnathostomata or (for bony fish) Osteichthyes, also contains the clade of tetrapods (four-limbed vertebrates, mostly terrestrial), which are not considered to be fish.[8][9][10] Some tetrapods, such as cetaceans and ichthyosaurs, have secondarily acquired a fish-like body shape through convergent evolution.[11] Fishes of the World comments that "it is increasingly widely accepted that tetrapods, including ourselves, are simply modified bony fishes, and so we are comfortable with using the taxon Osteichthyes as a clade, which now includes all tetrapods".[10] The cladogram shows clades traditionally considered as "fishes" (cyan line), along with the tetrapods. Extinct groups are marked with a dagger (†).
| Vertebrata/ |
|
"Fishes" | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Craniata |
Taxonomy
Fishes are a paraphyletic group and for this reason, the class Pisces seen in older reference works is no longer used in formal classifications. Traditional classification divides fish into three extant classes (Agnatha, Chondrichthyes, and Osteichthyes), and with extinct forms sometimes classified within those groups, sometimes as their own classes.[14]
Fish account for more than half of vertebrate species. As of 2016, there are over 32,000 described species of bony fish, over 1,100 species of cartilaginous fish, and over 100 hagfish and lampreys. A third of these fall within the nine largest families; from largest to smallest, these are Cyprinidae, Gobiidae, Cichlidae, Characidae, Loricariidae, Balitoridae, Serranidae, Labridae, and Scorpaenidae. About 64 families are monotypic, containing only one species.[10]
Diversity
-
Largest: whale shark
-
Smallest: Paedocypris progenetica (among others)
A typical fish is ectothermic, has a streamlined body for rapid swimming, extracts oxygen from water using gills, has two sets of paired fins, one or two dorsal fins, an anal fin and a tail fin, jaws, skin covered with scales, and lays eggs. Each criterion has exceptions, creating a wide diversity in body shape and way of life.[15]
Streamlining and swimming performance varies from fish such as tuna, salmon, and jacks that can cover 10–20 body-lengths per second to species such as eels and rays that swim no more than 0.5 body-lengths per second.[16] Fish range in size from the huge 16-metre (52 ft) whale shark to some tiny teleosts only 8-millimetre (0.3 in), such as the cyprinid Paedocypris progenetica[17] and the stout infantfish.[18]
The biodiversity of extant fish is unevenly distributed among the various groups, with teleosts making up 96% of fish species.[10] The cladogram[19] shows the evolutionary relationships of all groups of living fishes (with their respective diversity[10][20]) and the four-limbed vertebrates (tetrapods).[21]
| Vertebrates |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ecology

Fish species are roughly divided equally between marine (oceanic) and freshwater ecosystems. Coral reefs in the Indo-Pacific constitute the center of diversity for marine fishes, whereas continental freshwater fishes are most diverse in large river basins of tropical rainforests, especially the Amazon, Congo, and Mekong basins. More than 5,600 fish species inhabit Neotropical freshwaters alone, such that Neotropical fishes represent about 10% of all vertebrate species on the Earth. Exceptionally rich sites in the Amazon basin, such as Cantão State Park, can contain more freshwater fish species than occur in all of Europe.[22]
Fish are abundant in most bodies of water. They can be found in nearly all aquatic environments, from high mountain streams (e.g., char and gudgeon) to the abyssal and even hadal depths of the deepest oceans (e.g., cusk-eels and snailfish), although none have been found in the deepest 25% of the ocean.[23] With 34,300 described species, fish exhibit greater species diversity than any other group of vertebrates.[24] The deepest living fish in the ocean so far found is a cusk-eel, Abyssobrotula galatheae, recorded at the bottom of the Puerto Rico trench at 8,370 m (27,460 ft).[25][26]
In terms of temperature, Jonah's icefish live in cold[a] waters of the Southern Ocean, including under the Filchner–Ronne Ice Shelf at a latitude of 79°S,[28] while desert pupfish live in desert springs, streams, and marshes, sometimes highly saline, with water temperatures as high as 36 C.[29][30]
A few fish live mostly on land or lay their eggs on land near water.[31] Mudskippers feed and interact with one another on mudflats and go underwater to hide in their burrows.[32] A single undescribed species of Phreatobius has been called a true "land fish" as this worm-like catfish strictly lives among waterlogged leaf litter.[33][34] Cavefish of multiple species live in underground lakes, underground rivers or aquifers.[35]
Anatomy and physiology
Respiration
Gills
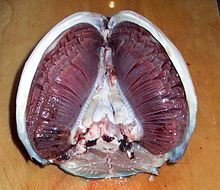
Most fish exchange gases using gills on either side of the pharynx. Gills consist of threadlike structures called filaments. Each filament contains a capillary network that provides a large surface area for exchanging oxygen and carbon dioxide. Fish exchange gases by pulling oxygen-rich water through their mouths and pumping it over their gills. In some fish, capillary blood flows in the opposite direction to the water, causing countercurrent exchange. The gills push the oxygen-poor water out through openings in the sides of the pharynx. Some fish, like sharks and lampreys, possess multiple gill openings. However, bony fish have a single gill opening on each side. This opening is hidden beneath a protective bony cover called an operculum. Juvenile bichirs have external gills, a primitive feature shared with larval amphibians.
Air breathing
Fish from multiple groups can live out of the water for extended periods. Amphibious fish such as the mudskipper can move about on land and live in oxygen-depleted water. The skin of anguillid eels may absorb oxygen directly. The buccal cavity of the electric eel may breathe air. Catfish of the families Loricariidae, Callichthyidae, and Scoloplacidae absorb air through their digestive tracts.[36] Bichirs and lungfish (except for the Australian lungfish), have paired lungs similar to those of tetrapods; they have to surface to gulp fresh air through the mouth and pass spent air out through the gills. Gar and bowfin have a vascularized swim bladder that functions in the same way. Loaches, trahiras, and many catfish breathe by passing air through the gut. Mudskippers breathe by absorbing oxygen across the skin (similar to frogs). Some fish have evolved accessory breathing organs: labyrinth fish such as gouramis and bettas have a labyrinth organ above the gills, while snakeheads, pikeheads, and airbreathing catfish have similar structures. Some air-breathing fish are able to survive in damp burrows for weeks without water, entering a state of aestivation (summertime hibernation) until water returns. Air breathing fish can be divided into obligate air breathers and facultative air breathers. Obligate air breathers, such as the African lungfish, must breathe air periodically or they suffocate. Facultative air breathers, such as some catfish, only breathe air if they need to and will otherwise rely on their gills for oxygen. Most air breathing fish are facultative air breathers; they avoid the energetic cost of rising to the surface and the fitness cost of exposure to surface predators.[36]
Circulation

Fish have a closed-loop circulatory system. The heart pumps the blood in a single loop throughout the body, with two chambers; for comparison, the mammal heart has two loops through the body and four chambers.[37] The first part is the sinus venosus, a thin-walled sac that collects blood from the fish's veins before allowing it to flow to the second part, the atrium, which is a large muscular chamber. The atrium serves as a one-way antechamber, sends blood to the third part, ventricle. The ventricle is another thick-walled, muscular chamber and it pumps the blood, first to the fourth part, bulbus arteriosus, a large tube, and then out of the heart. The bulbus arteriosus connects to the aorta, through which blood flows to the gills for oxygenation.
Digestion
Jaws allow fish to eat a wide variety of food, including plants and other organisms. Fish ingest food through the mouth and break it down in the esophagus. In the stomach, food is further digested and, in many fish, processed in finger-shaped pouches called pyloric caeca, which secrete digestive enzymes and absorb nutrients. Organs such as the liver and pancreas add enzymes and various chemicals as the food moves through the digestive tract. The intestine completes the process of digestion and nutrient absorption.
Excretion
Most fish release their nitrogenous wastes as ammonia. This may be excreted through the gills or filtered by the kidneys.
Saltwater fish tend to lose water by osmosis; their kidneys return water to the body, and produce a concentrated urine. The reverse happens in freshwater fish: they tend to gain water osmotically, and produce a dilute urine. Some fish have kidneys able to operate in both freshwater to saltwater.
Brain

Fish have small brains relative to body size compared with other vertebrates, typically one-fifteenth the brain mass of a similarly-sized bird or mammal.[38] However, some fish have relatively large brains, notably mormyrids and sharks, which have brains about as large for their body weight as birds and marsupials.[39] Fish brains are divided into several regions. At the front are the olfactory lobes, a pair of structures that receive and process signals from the nostrils via the two olfactory nerves.[38] The olfactory lobes are very large in fish that hunt primarily by smell, such as hagfish, sharks, and catfish. Behind the olfactory lobes is the two-lobed telencephalon, the structural equivalent to the cerebrum in higher vertebrates. In fish the telencephalon is concerned mostly with olfaction. Together these structures form the forebrain.[38] Connecting the forebrain to the midbrain is the diencephalon (in the diagram, this structure is below the optic lobes and consequently not visible). The diencephalon performs functions associated with hormones and homeostasis.[38] The pineal body lies just above the diencephalon. This structure detects light, maintains circadian rhythms, and controls color changes.[38] The midbrain (or mesencephalon) contains the two optic lobes. These are very large in species that hunt by sight, such as rainbow trout and cichlids.[38] The hindbrain (or metencephalon) is particularly involved in swimming and balance.[38] The cerebellum is a single-lobed structure that is typically the biggest part of the brain.[38] Hagfish and lampreys have relatively small cerebellae, while the mormyrid cerebellum is massive and apparently involved in their electrical sense.[38] The brain stem or myelencephalon is the brain's posterior.[38] As well as controlling some muscles and body organs, in bony fish at least, the brain stem governs respiration and osmoregulation.[38]
Sensory systems
The lateral line system is a network of sensors in the skin which detects gentle currents and vibrations, and senses the motion of nearby fish, whether predators or prey.[40] This can be considered both a sense of touch and of hearing. Blind cave fish navigate almost entirely through the sensations from their lateral line system.[41] Some fish, such as catfish and sharks, have the ampullae of Lorenzini, electroreceptors that detect weak electric currents on the order of millivolt.[42] Vision in fishes is an important sensory system. Fish eyes are similar to those of terrestrial vertebrates like birds and mammals, but have a more spherical lens. Their retinas generally have both rods and cones (for scotopic and photopic vision); most species have colour vision. Some fish can see ultraviolet, while others can see polarized light. Amongst jawless fish, the lamprey has well-developed eyes, while the hagfish has only primitive eyespots.[43] Hearing too is an important sensory system in fish. Fish sense sound using their lateral lines and otoliths in their ears, inside their heads. Some can detect sound through the swim bladder.[44] Some fish, including salmon, are capable of magnetoreception; when the axis of a magnetic field is changed around a circular tank of young fish, they reorient themselves in line with the field.[45][46] The mechanism of fish magnetoreception remains unknown;[47] experiments in birds imply a quantum radical pair mechanism.[48]
Cognition
The cognitive capacities of fish include self-awareness, as seen in mirror tests. Manta rays and wrasses placed in front of a mirror repeatedly check whether their reflection's behavior mimics their body movement.[49][50][51] Choerodon wrasse, archerfish, and Atlantic cod can solve problems and invent tools.[52] The monogamous cichlid Amatitlania siquia exhibits pessimistic behavior when prevented from being with its partner.[53] Fish orient themselves using landmarks; they may use mental maps based on multiple landmarks. Fish behavior in mazes reveals that they possess spatial memory and visual discrimination.[54] Fish have pain and fear responses; toadfish grunt when electrically shocked and over time come to grunt at the mere sight of an electrode.[55] Rainbow trout rock their bodies and rub their lips when injected with bee venom and acetic acid, apparently attempting to relieve pain.[56][57] The wrasse's neurons fired in a pattern resembling human neuronal patterns.[57] The claims have been called flawed as they do not prove that fish possess conscious awareness, especially not human-like awareness,[58] and their brains are very different.[59]
Locomotion


Most fish move by alternately contracting paired sets of muscles on either side of the backbone. These contractions form S-shaped curves that move down the body. As each curve reaches the back fin, backward force is applied to the water, and in conjunction with the fins, moves the fish forward. The fish's fins function like an airplane's flaps. Fins also increase the tail's surface area, increasing speed.[60]
The streamlined body of the fish decreases the amount of friction from the water. Since body tissue is denser than water, fish must compensate for the difference or they will sink. Many bony fish have an internal organ called a swim bladder that adjusts their buoyancy through manipulation of gases. The scales of fish originate from the mesoderm (skin); they may be similar in structure to teeth.
Electrogenesis

Electric fish such as elephantfishes, the African knifefish, and electric eels have some of their muscles adapted to generate electric fields. They use the field to locate and identify objects such as prey in the waters around them, which may be turbid or dark.[62] Strongly electric fish like the electric eel can in addition use their electric organs to generate shocks powerful enough to stun their prey.[63]
Endothermy
Most fish are exclusively cold-blooded or ectothermic. However, the Scombroidei are warm-blooded (endothermic), including the billfishes and tunas.[64] The opah, a lampriform, uses whole-body endothermy, generating heat with its swimming muscles to warm its body while countercurrent exchange minimizes heat loss.[65] Among the cartilaginous fishes, sharks of the families Lamnidae (such as the great white shark) and Alopiidae (thresher sharks) are endothermic. The degree of endothermy varies from the billfishes, which warm only their eyes and brain, to the bluefin tuna and the porbeagle shark, which maintain body temperatures more than 20 °C (68 °F) above the ambient water.[64][66][67]
Reproduction

The primary reproductive organs are testicles and ovaries. In most species, gonads are paired organs of similar size, which can be partially or totally fused.[68] Some fish have secondary organs that increase reproductive fitness.
In terms of spermatogonia distribution, the structure of teleosts testes has two types: in the most common, spermatogonia occur all along the seminiferous tubules, while in atherinomorph fish they are confined to the distal portion of these structures. Fish can present cystic or semi-cystic spermatogenesis in relation to the release phase of germ cells in cysts to the seminiferous tubules lumen.[68]
Fish ovaries may be of three types: gymnovarian, secondary gymnovarian or cystovarian. In the first type, the oocytes are released directly into the coelomic cavity and then enter the ostium, then through the oviduct and are eliminated. Secondary gymnovarian ovaries shed ova into the coelom from which they go directly into the oviduct. In the third type, the oocytes are conveyed to the exterior through the oviduct.[69] Gymnovaries are the primitive condition found in lungfish, sturgeon, and bowfin. Cystovaries characterize most teleosts, where the ovary lumen has continuity with the oviduct.[68] Secondary gymnovaries are found in salmonids and a few other teleosts.
Oogonia development in teleosts fish varies according to the group, and the determination of oogenesis dynamics allows the understanding of maturation and fertilization processes. Changes in the nucleus, ooplasm, and the surrounding layers characterize the oocyte maturation process.[68] Postovulatory follicles are structures formed after oocyte release; they do not have endocrine function, present a wide irregular lumen, and are rapidly reabsorbed in a process involving the apoptosis of follicular cells. A degenerative process called follicular atresia reabsorbs vitellogenic oocytes not spawned. This process can also occur, but less frequently, in oocytes in other development stages.[68]
Some fish, like the California sheephead, are hermaphrodites, having both testes and ovaries either at different phases in their life cycle or, as in hamlets, have them simultaneously.
Over 97% of fish are oviparous,[70] that is, the eggs develop outside the mother's body. Examples of oviparous fish include salmon, goldfish, cichlids, tuna, and eels. In the majority of these species, fertilisation takes place outside the mother's body, with the male and female fish shedding their gametes into the surrounding water. However, a few oviparous fish practice internal fertilization, with the male using some sort of intromittent organ to deliver sperm into the genital opening of the female, most notably the oviparous sharks, such as the horn shark, and oviparous rays, such as skates. In these cases, the male is equipped with a pair of modified pelvic fins known as claspers.
Marine fish can produce high numbers of eggs which are often released into the open water column. The eggs have an average diameter of 1 millimetre (0.04 in).
-
Egg of lamprey
-
Egg of catshark (mermaids' purse)
-
Egg of bullhead shark
-
Egg of chimaera
The newly hatched young of oviparous fish are called larvae. They are usually poorly formed, carry a large yolk sac (for nourishment), and do not resemble juvenile or adult fish. The larval period in oviparous fish is usually only someweeks, and larvae rapidly grow and change in structure to become juveniles. During this transition, larvae must switch from their yolk sac to feeding on zooplankton prey, a process which depends on typically inadequate zooplankton density, starving many larvae.
In ovoviviparous fish the eggs develop inside the mother's body after internal fertilization but receive little or no nourishment directly from the mother, depending instead on the yolk. Each embryo develops in its own egg. Familiar examples of ovoviviparous fish include guppies, angel sharks, and coelacanths.
Some species of fish are viviparous. In such species the mother retains the eggs and nourishes the embryos. Typically, viviparous fish have a structure analogous to the placenta seen in mammals connecting the mother's blood supply with that of the embryo. Examples of viviparous fish include the surf-perches, splitfins, and lemon shark. Some viviparous fish exhibit oophagy, in which the developing embryos eat other eggs produced by the mother. This has been observed primarily among sharks, such as the shortfin mako and porbeagle, but is known for a few bony fish as well, such as the halfbeak Nomorhamphus ebrardtii.[71] Intrauterine cannibalism is an even more unusual mode of vivipary, in which the largest embryos eat weaker and smaller siblings. This behavior is also most commonly found among sharks, such as the grey nurse shark, but has also been reported for Nomorhamphus ebrardtii.[71]
Behavior
Shoaling and schooling

An assemblage of fish merely using some localised resource such as food or nesting sites is called an aggregation. A shoal is a loosely organised group where each fish swims and forages independently but is attracted to other members of the group and adjusts its behaviour, such as swimming speed, so that it remains close to the other members of the group. A school is a much more tightly organised group, synchronising its swimming so that all fish move at the same speed and in the same direction.[73] Schooling is sometimes an antipredator adaptation, offering improved vigilance against predators. It is often more efficient to gather food by working as a group, and individual fish optimise their strategies by choosing to join or leave a shoal. When a predator has been noticed, prey fish respond defensively, resulting in collective shoal behaviours such as synchronised movements. Responses do not consist only of attempting to hide or flee; antipredator tactics include for example scattering and reassembling. Fish also aggregate in shoals to spawn.[72]
Communication
Fish communicate by transmitting sounds, acoustic signals, to each other. This is most often in the context of feeding, aggression or courtship.[74] The sounds emitted vary with the species and stimulus involved. Fish can produce either stridulatory sounds by moving components of the skeletal system, or can produce non-stridulatory sounds by manipulating specialized organs such as the swimbladder.[75]

Some fish produce sounds by rubbing or grinding their bones together. These sounds are stridulatory. In Haemulon flavolineatum, the French grunt fish, as it produces a grunting noise by grinding its teeth together, especially when in distress. The grunts are at a frequency of around 700 Hz, and last approximately 47 milliseconds.[75] The longsnout seahorse, Hippocampus reidi produces two categories of sounds, 'clicks' and 'growls', by rubbing their coronet bone across the grooved section of their neurocranium.[76] Clicks are produced during courtship and feeding, and the frequencies of clicks were within the range of 50 Hz-800 Hz. The frequencies are at the higher end of the range during spawning, when the female and male fishes were less than fifteen centimeters apart. Growls are produced when the H. reidi are stressed. The 'growl' sounds consist of a series of sound pulses and are emitted simultaneously with body vibrations.[77]
Some fish species create noise by engaging specialized muscles that contract and cause swimbladder vibrations. Oyster toadfish produce loud grunts by contracting sonic muscles along the sides of the swim bladder.[78] Female and male toadfishes emit short-duration grunts, often as a fright response.[79] In addition to short-duration grunts, male toadfishes produce "boat whistle calls".[80] These calls are longer in duration, lower in frequency, and are primarily used to attract mates.[80] The sounds emitted by the O. tao have frequency range of 140 Hz to 260 Hz.[80] The frequencies of the calls depend on the rate at which the sonic muscles contract.[81][78]
The red drum, Sciaenops ocellatus, produces drumming sounds by vibrating its swimbladder. Vibrations are caused by the rapid contraction of sonic muscles that surround the dorsal aspect of the swimbladder. These vibrations result in repeated sounds with frequencies from 100 to >200 Hz. S. ocellatus produces different calls depending on the stimuli involved, such as courtship or a predator's attack. Females do not produce sounds, and lack sound-producing (sonic) muscles.[82]
Defense against disease

Like other animals, fish suffer from diseases and parasites. To prevent disease they have a variety of defenses. Non-specific defenses include the skin and scales, as well as the mucus layer secreted by the epidermis that traps and inhibits the growth of microorganisms. If pathogens breach these defenses, fish can develop an inflammatory response that increases blood flow to the infected region and delivers white blood cells that attempt to destroy pathogens. Specific defenses respond to particular pathogens recognised by the fish's body, i.e., an immune response.[83]
Some species use cleaner fish to remove external parasites. The best known of these are the bluestreak cleaner wrasses of coral reefs in the Indian and Pacific oceans. These small fish maintain cleaning stations where other fish congregate and perform specific movements to attract the attention of the cleaners.[84] Cleaning behaviors have been observed in a number of fish groups, including an interesting case between two cichlids of the same genus, Etroplus maculatus, the cleaner, and the much larger Etroplus suratensis.[85]
Immune organs vary by type of fish. In jawless fish, true lymphoid organs are absent. These fish rely on regions of lymphoid tissue within other organs to produce immune cells. For example, erythrocytes, macrophages and plasma cells are produced in the anterior kidney and some areas of the gut (where granulocytes mature). They resemble primitive bone marrow in hagfish. Cartilaginous fish have a more advanced immune system with three specialized organs: the epigonal organs around the gonads, Leydig's organ within the esophagus, and a spiral valve in their intestine. These organs house typical immune cells such as lymphocytes. They also possess a thymus and a well-developed spleen where lymphocytes, plasma cells and macrophages develop and are stored. Chondrostean fish produce granulocytes in tissue near the meninges. Their heart is covered with tissue that contains lymphocytes and reticular cells, while erythrocytes, granulocytes, lymphocytes and macrophages develop in the kidney.[86]
The major immune tissues of bony fish include the kidney, which houses many different immune cells.[87] In addition, teleost fish possess a thymus, spleen and scattered immune areas within mucosal tissues (e.g. in the skin, gills, gut and gonads). Much like the mammalian immune system, teleost erythrocytes, neutrophils and granulocytes are believed to reside in the spleen whereas lymphocytes are the major cell type found in the thymus.[88][89] An unconfirmed report claims a lymphatic system similar to that in mammals in teleosts, presumably where naive T cells accumulate while waiting to encounter an antigen.[90]
B and T lymphocytes bearing immunoglobulins and T cell receptors respectively are found in all jawed fishes. Indeed, the adaptive immune system as a whole evolved in an ancestor of all jawed vertebrates.[91]
Conservation
The 2006 IUCN Red List names 1,173 fish species that are threatened with extinction.[92] Included are species such as Atlantic cod,[93] Devil's Hole pupfish,[94] coelacanths,[95] and great white sharks.[96] Because fish live underwater they are more difficult to study than terrestrial animals and plants, and information about fish populations is often lacking. However, freshwater fish seem particularly threatened because they often live in relatively small water bodies. For example, the Devil's Hole pupfish occupies only a single 3 by 6 metres (10 by 20 ft) pool.[97]
Overfishing

Overfishing is a major threat to edible fish such as cod and tuna.[98][99] Overfishing eventually causes population (known as stock) collapse because the survivors cannot produce enough young to replace those removed. Such commercial extinction does not mean that the species is extinct, merely that it can no longer sustain a fishery. A well-studied example of fishery collapse is the Pacific sardine Sadinops sagax caerulues fishery off the California coast. From a 1937 peak of 790,000 long tons (800,000 t) the catch steadily declined to only 24,000 long tons (24,000 t) in 1968, after which the fishery was no longer economically viable.[100]
Fisheries scientists and the fishing industry have different views on the resiliency of fisheries to intensive fishing. In places such as Scotland, Newfoundland, and Alaska the fishing industry is a major employer, so governments are predisposed to support it.[101][102] On the other hand, scientists and conservationists push for stringent protection, warning that many stocks could be wiped out within fifty years.[103][104]
Habitat destruction
A key stress on both freshwater and marine ecosystems is habitat degradation including water pollution, the building of dams, removal of water for use by humans, and the introduction of exotic species.[105] An example of a fish that has become endangered because of habitat change is the pallid sturgeon, a North American freshwater fish that lives in rivers damaged by human activity.[106]
Exotic species
Introduction of non-native species occurs in many habitats. The Mediterranean Sea has become a major 'hotspot' of exotic invaders since the opening of the Suez Canal in 1869. A thousand marine species of all sorts – fishes, seaweeds, invertebrates – originating from the Red Sea and more broadly from the Indo-Pacific have crossed the Canal from south to north to settle in the eastern Mediterranean Basin. Nowadays many of these tropical or Lessepsian migrants, have extended their range towards the west, obviously favoured by the general warming of the Mediterranean. The resulting change in biodiversity is without precedent in human memory and is accelerating: a long-term cross-Basin survey engaged by the Mediterranean Science Commission recently documented that in just twenty years, from 2001 till 2021, no less than 107 alien fish species have reached the Mediterranean from both the tropical Atlantic and the Red Sea, which is more than the total recorded during the whole 130 preceding years.[107]
Another mode of introduction for marine species is transport across thousands of kms on ship hulls or in ballast waters. Examples abound of marine organisms being transported in ballast water, among them the invasive comb jelly Mnemiopsis leidyi, the dangerous bacterium Vibrio cholerae, or the fouling zebra mussel. The Mediterranean and Black Seas, with their high volume shipping from exotic harbors, are particularly impacted by this problem.[108]
Deliberate introductions of species with market potential are another frequent vector. A well-studied example is the introduction of the Nile perch into Lake Victoria in the 1960s. This predatory fish gradually exterminated the lake's 500 endemic cichlid species. Some of them now survive in captive breeding programmes, but others are probably extinct.[109]
Importance to humans
Economic

Throughout history, humans have used fish as a food source for dietary protein. Historically and today, most fish harvested for human consumption has come by means of catching wild fish. However, fish farming, which has been practiced since about 3,500 BCE in ancient China,[110] is becoming increasingly important in many nations. Overall, about one-sixth of the world's protein is estimated to be provided by fish.[111] Fishing is accordingly a large global business which provides income for millions of people.[111] The Environmental Defense Fund has a guide on which fish are safe to eat, given the state of pollution in today's world, and which fish are obtained in a sustainable way.[112] As of 2020, over 65 million tonnes (Mt) of marine fish and 10 Mt of freshwater fish were captured, while some 50 Mt of fish, mainly freshwater, were farmed. Of the marine species captured in 2020, anchoveta represented 4.9 Mt, Alaska pollock 3.5 Mt, skipjack tuna 2.8 Mt, and Atlantic herring and yellowfin tuna 1.6 Mt each; eight more species had catches over 1 Mt.[113]
Recreation
Fish have been recognized as a source of beauty for almost as long as used for food, appearing in cave art, being raised as ornamental fish in ponds, and displayed in aquariums in homes, offices, or public settings. Recreational fishing is fishing primarily for pleasure or competition; it can be contrasted with commercial fishing, which is fishing for profit, or artisanal fishing, which is fishing primarily for food. The most common form of recreational fishing is done with a rod, reel, line, hooks, and any one of a wide range of baits. Recreational fishing is particularly popular in North America and Europe and state, provincial, and federal government agencies actively management target fish species.[114][115]
Culture
Fish themes have symbolic significance in many religions. In ancient Mesopotamia, fish offerings were made to the gods from the very earliest times.[116] Fish were also a major symbol of Enki, the god of water.[116] Fish frequently appear as filling motifs in cylinder seals from the Old Babylonian (c. 1830 BC – c. 1531 BC) and Neo-Assyrian (911–609 BC) periods.[116] Starting during the Kassite Period (c. 1600 BC – c. 1155 BC) and lasting until the early Persian Period (550–30 BC), healers and exorcists dressed in ritual garb resembling the bodies of fish.[116] During the Seleucid Period (312–63 BC), the legendary Babylonian culture hero Oannes, described by Berossus, was said to have dressed in the skin of a fish.[116] Fish were sacred to the Syrian goddess Atargatis[117] and, during her festivals, only her priests were permitted to eat them.[117] In the Book of Jonah, the central figure, a prophet named Jonah, is swallowed by a giant fish after being thrown overboard by the crew of the ship he is travelling on.[118] Early Christians used the ichthys, a symbol of a fish, to represent Jesus,[117][119] because the Greek word for fish, ΙΧΘΥΣ Ichthys, could be used as an acronym for "Ίησοῦς Χριστός, Θεοῦ Υἱός, Σωτήρ" (Iesous Christos, Theou Huios, Soter), meaning "Jesus Christ, Son of God, Saviour".[117][119] Among the deities said to take the form of a fish are Ika-Roa of the Polynesians, Dagon of various ancient Semitic peoples, the shark-gods of Hawaiʻi and Matsya of the Hindus. The astrological symbol Pisces is based on a constellation of the same name, but there is also a second fish constellation in the night sky, Piscis Austrinus.[120]
Fish feature prominently in art, in movies such as Finding Nemo and books such as The Old Man and the Sea. Large fish, particularly sharks, have frequently been the subject of horror movies and thrillers, notably the novel Jaws, in turn parodied in Shark Tale and Snakehead Terror. Piranhas are shown in a similar light to sharks in films such as Piranha.[121]
-
The Fishmonger's Shop, Bartolomeo Passerotti, 1580s
-
Goldfish by Henri Matisse, 1912
See also
- Catch and release
- Deep sea fish
- Fish acute toxicity syndrome
- Fish anatomy
- Fish development
- Forage fish
- Ichthyology
- List of fish common names
- List of fish families
- Marine biology
- Marine vertebrates
- Mercury in fish
- Otolith (Bone used for determining the age of a fish)
- Pregnancy (fish)
- Seafood
- Shoaling and schooling
- Walking fish
Notes
- ^ The temperature is often around 0 C. The freezing point of seawater at the surface is -1.85 C, falling to -2.62 C at a depth of 1000 metres. However, the water can be supercooled somewhat below these temperatures.[27]
References
- ^ "DWDS – Digitales Wörterbuch der deutschen Sprache". DWDS (in German). Archived from the original on 31 July 2020. Retrieved 21 January 2023.
- ^ Winfred Philipp Lehmann, Helen-Jo J. Hewitt, Sigmund Feist, A Gothic etymological dictionary, 1986, s.v. fisks p. 118
- ^ "fish, n.1", OED Online, Oxford University Press, archived from the original on 17 March 2023, retrieved 21 January 2023
- ^ Carl Darling Buck, A Dictionary of Selected Synonyms in the Principal Indo-European Languages, 1949, s.v., section 3.65, p. 184
- ^ a b Donoghue, Philip C. J.; Purnell, Mark A. (2009). "The Evolutionary Emergence of Vertebrates From Among Their Spineless Relatives". Evolution: Education and Outreach. 2 (2): 204–212. doi:10.1007/s12052-009-0134-3. ISSN 1936-6426.
- ^ Shu, D. G.; Morris, S. C.; Han, J.; Zhang, Z. F.; Yasui, K.; Janvier, P.; Chen, L.; Zhang, X. L.; Liu, J. N.; Li, Y.; Liu, H.-Q. (2003). "Head and backbone of the Early Cambrian vertebrate Haikouichthys". Nature. 421 (6922): 526–529. Bibcode:2003Natur.421..526S. doi:10.1038/nature01264. PMID 12556891. S2CID 4401274.
- ^ "Monster fish crushed opposition with strongest bite ever". Smh.com.au. 30 November 2006. Archived from the original on 2 April 2013. Retrieved 26 February 2013.
- ^ "Zoology" (PDF). Archived (PDF) from the original on 28 June 2021. Retrieved 19 October 2018.
- ^ Greene, Harry W. (1 January 1998). "We are primates and we are fish: Teaching monophyletic organismal biology". Integrative Biology. 1 (3): 108–111. doi:10.1002/(sici)1520-6602(1998)1:3<108::aid-inbi5>3.0.co;2-t. ISSN 1520-6602.
- ^ a b c d e Nelson, Joseph S. (2016). "Taxonomic Diversity". Fishes of the World. John Wiley & Sons. p. 3. ISBN 978-1-118-34233-6.
- ^ Davis, R. W. (2019). "Return to the Sea: The Evolution of Marine Mammals". In Davis, R. W. (ed.). Marine Mammals: Adaptations for an Aquatic Life. New York: Springer International Publishing. pp. 7–27. ISBN 978-3-3199-8278-6.
- ^
 Giles, Sam; Friedman, Matt; Brazeau, Martin D. (12 January 2015). "Osteichthyan-like cranial conditions in an Early Devonian stem gnathostome". Nature. 520 (7545): 82–85. Bibcode:2015Natur.520...82G. doi:10.1038/nature14065. ISSN 1476-4687. PMC 5536226. PMID 25581798.
Giles, Sam; Friedman, Matt; Brazeau, Martin D. (12 January 2015). "Osteichthyan-like cranial conditions in an Early Devonian stem gnathostome". Nature. 520 (7545): 82–85. Bibcode:2015Natur.520...82G. doi:10.1038/nature14065. ISSN 1476-4687. PMC 5536226. PMID 25581798.
- ^ Davis, S; Finarelli, J; Coates, M (2012). "Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes". Nature. 486 (7402): 247–250. Bibcode:2012Natur.486..247D. doi:10.1038/nature11080. PMID 22699617. S2CID 4304310.
- ^ Benton, M.J. (1998). "The quality of the fossil record of vertebrates". In Donovan, S.K.; Paul, C.R.C. (eds.). The adequacy of the fossil record. Wiley. pp. 269–303, Fig. 2.
- ^ Helfman, Collette & Facey 1997, pp. 3, 33–36.
- ^ Helfman, Collette & Facey 1997, p. 103.
- ^ Kottelat, Maurice; Britz, Ralf; Heok Hui, Tan; Witte, Kai-Erik (2005). "Paedocypris, a new genus of Southeast Asian cyprinid fish with a remarkable sexual dimorphism,comprises the world's smallest vertebrate" (PDF). Proceedings of the Royal Society B. 273. The Royal Society: 895–899. doi:10.1098/rspb.2005.3419. PMC 1560243. PMID 16627273. Archived from the original (PDF) on 12 July 2009. Retrieved 26 October 2012.
- ^ Froese, Rainer; Pauly, Daniel (eds.). "Schindleria brevipinguis". FishBase. September 2017 version.
- ^ Friedman, Matt; Sallan, Lauren Cole (June 2012). "Five hundred million years of extinczion and recovery: A Phanerozoic survey of large-scale diversity patterns in fishes". Palaeontology. 55 (4): 707–742. Bibcode:2012Palgy..55..707F. doi:10.1111/j.1475-4983.2012.01165.x. S2CID 59423401.
- ^ Brownstein; et al. (27 July 2022). "Hidden species diversity in a living fossil vertebrate" (PDF). Biology Letters. 18 (11). bioRxiv 10.1101/2022.07.25.500718. doi:10.1098/rsbl.2022.0395. PMC 9709656. PMID 36448369. S2CID 251162051.
- ^ "Summary Statistics". IUCN Red List of Threatened Species. 2023.1. Retrieved 5 February 2024. Table 1a: Number of species evaluated in relation to the overall number of described species, and numbers of threatened species by major groups of organisms
- ^ "Estudo das Espécies Ícticas do Parque Estadual do Cantão". central3.to.gov.br. Archived from the original on 15 August 2022. Retrieved 21 January 2023.
- ^ Yancey, P.H.; Gerringer, M.E.; Drazen, J.C.; Rowden, A.A.; Jamieson, A. (2014). "Marine fish may be biochemically constrained from inhabiting the deepest ocean depths". Proc Natl Acad Sci USA. 111 (12): 4461–4465. Bibcode:2014PNAS..111.4461Y. doi:10.1073/pnas.1322003111. PMC 3970477. PMID 24591588.
- ^ "FishBase Search". FishBase. March 2020. Archived from the original on 3 March 2020. Retrieved 19 March 2020.
- ^ Nielsen, Jørgen G. (1998). Paxton, J.R.; Eschmeyer, W.N. (eds.). Encyclopedia of Fishes. San Diego: Academic Press. p. 134. ISBN 0-12-547665-5.
- ^ "What is the deepest-living fish?". Australian Museum. 23 December 2014. Retrieved 18 September 2015.
- ^ Haumann, F. Alexander; Moorman, Ruth; Riser, Stephen C.; Smedsrud, Lars H.; Maksym, Ted; Wong, Annie P.S.; Wilson, Earle A.; Drucker, Robert; Talley, Lynne D.; Johnson, Kenneth S.; Key, Robert M.; Sarmiento, Jorge L. (28 October 2020). "Supercooled Southern Ocean Waters". Geophysical Research Letters. 47 (20). doi:10.1029/2020GL090242.
- ^ Purser, Autun; Hehemann, Laura; Boehringer, Lilian; Tippenhauer, Sandra; Wege, Mia; Bornemann, Horst; et al. (2022). "A vast icefish breeding colony discovered in the Antarctic". Current Biology. 32 (4): 842–850.e4. doi:10.1016/j.cub.2021.12.022. hdl:2263/90796. PMID 35030328. S2CID 245936769.
- ^ Marsh, Paul C.; Sada, Donald W (1993). "Desert Pupfish (Cyprinodon macularius) Recovery Plan" (PDF). United States Fish and Wildlife Service. Archived (PDF) from the original on 17 October 2011.
- ^ Shrode, Joy B.; Gerking, Shelby D. (1977). "Effects of Constant and Fluctuating Temperatures on Reproductive Performance of a Desert Pupfish, Cyprinodon n. nevadensis". Physiological Zoology. 50 (1): 1–10. doi:10.1086/physzool.50.1.30155710. ISSN 0031-935X. S2CID 82166135.
- ^ Martin, K.L.M. (2014). Beach-Spawning Fishes: Reproduction in an Endangered Ecosystem. CRC Press. ISBN 978-1-4822-0797-2.
- ^ Froese, Rainer; Pauly, Daniel (eds.). "Periophthalmus barbarus". FishBase. November 2006 version.
- ^ Planet Catfish. "Cat-eLog: Heptapteridae: Phreatobius: Phreatobius sp. (1)". Planet Catfish. Archived from the original on 23 October 2006. Retrieved 26 November 2006.
- ^ Henderson, P.A.; Walker, I. (1990). "Spatial organization and population density of the fish community of the litter banks within a central Amazonian blackwater stream". Journal of Fish Biology. 37 (3): 401–411. Bibcode:1990JFBio..37..401H. doi:10.1111/j.1095-8649.1990.tb05871.x.
- ^ Helfman, G.S. (2007). Fish Conservation: A Guide to Understanding and Restoring Global Aquatic Biodiversity and Fishery Resources. Island Press. pp. 41–42. ISBN 978-1-55963-595-0.
- ^ a b Armbruster, Jonathan W. (1998). "Modifications of the Digestive Tract for Holding Air in Loricariid and Scoloplacid Catfishes" (PDF). Copeia. 1998 (3): 663–675. doi:10.2307/1447796. JSTOR 1447796. Archived from the original (PDF) on 26 March 2009. Retrieved 25 June 2009.
- ^ Setaro, John F. (1999). Circulatory System. Microsoft Encarta 99.
- ^ a b c d e f g h i j k Helfman, Collette & Facey 1997, pp. 48–49.
- ^ Helfman, Collette & Facey 1997, p. 191.
- ^ Orr, James (1999). Fish. Microsoft Encarta 99. ISBN 978-0-8114-2346-5.
- ^ Godfrey-Smith, Peter (2020). "Kingfish". Metazoa. New York: Farrar, Straus, and Giroux. ISBN 9780374207946.
- ^ Albert, J.S., and W.G.R. Crampton. 2005. "Electroreception and electrogenesis". pp. 431–472 in The Physiology of Fishes, 3rd ed. D.H. Evans and J.B. Claiborne (eds.). CRC Press.
- ^ Campbell, Neil A.; Reece, Jane B. (2005). Biology (Seventh ed.). San Francisco: Benjamin Cummings.
- ^ Hawkins, A. D. (1981). "6. The Hearing Abilities of Fish". In Tavolga, William N.; Popper, Arthur N.; Fay, Richard R. (eds.). Hearing and Sound Communication in Fishes. Springer. pp. 109–138. ISBN 978-1-4615-7188-9.
- ^ Quinn, Thomas P. (1980). "Evidence for celestial and magnetic compass orientation in lake migrating sockeye salmon fry". Journal of Comparative Physiology A. 137 (3): 243–248. doi:10.1007/bf00657119. S2CID 44036559.
- ^ Taylor, P. B. (May 1986). "Experimental evidence for geomagnetic orientation in juvenile salmon, Oncorhynchus tschawytscha Walbaum". Journal of Fish Biology. 28 (5): 607–623. doi:10.1111/j.1095-8649.1986.tb05196.x.
- ^ Formicki, Krzysztof; Korzelecka‐Orkisz, Agata; Tański, Adam (2019). "Magnetoreception in fish". Journal of Fish Biology. 95 (1): 73–91. doi:10.1111/jfb.13998. ISSN 0022-1112.
- ^ Hore, Peter J.; Mouritsen, Henrik (April 2022). "The Quantum Nature of Bird Migration". Scientific American: 24–29.
- ^ Ari, Csilla; D’Agostino, Dominic P. (1 May 2016). "Contingency checking and self-directed behaviors in giant manta rays: Do elasmobranchs have self-awareness?". Journal of Ethology. 34 (2): 167–174. doi:10.1007/s10164-016-0462-z. ISSN 1439-5444. S2CID 254134775. Archived from the original on 17 March 2023. Retrieved 21 January 2023.
- ^ Kohda, Masanori; Hotta, Takashi; Takeyama, Tomohiro; Awata, Satoshi; Tanaka, Hirokazu; Asai, Jun-ya; Jordan, L. Alex (21 August 2018). "Cleaner wrasse pass the mark test. What are the implications for consciousness and self-awareness testing in animals?". PLOS Biology: 397067. doi:10.1101/397067. S2CID 91375693. Archived from the original on 23 January 2020. Retrieved 26 March 2019.
- ^ "Scientists find some fish can 'recognise themselves' in mirror". the Guardian. 7 February 2019. Archived from the original on 21 January 2023. Retrieved 21 January 2023.
- ^ Fishes Use Problem-Solving and Invent Tools Archived 17 March 2023 at the Wayback Machine- article at Scientific American Archived 18 May 2020 at the Wayback Machine
- ^ Laubu, Chloé; Louâpre, Philippe; Dechaume-Moncharmont, François-Xavier (2019). "Pair-bonding influences affective state in a monogamous fish species". Proc. R. Soc. B. 286 (1904). 20190760. doi:10.1098/rspb.2019.0760. PMC 6571461. PMID 31185864.
- ^ Sciences, Journal of Undergraduate Life. "Appropriate maze methodology to study learning in fish" (PDF). Archived from the original (PDF) on 6 July 2011. Retrieved 28 May 2009.
- ^ Dunayer, Joan, "Fish: Sensitivity Beyond the Captor's Grasp," The Animals' Agenda, July/August 1991, pp. 12–18
- ^ Kirby, Alex (30 April 2003). "Fish do feel pain, scientists say". BBC News. Archived from the original on 15 February 2009. Retrieved 4 January 2010.
- ^ a b Grandin, Temple; Johnson, Catherine (2005). Animals in Translation. New York City: Scribner. pp. 183–184. ISBN 978-0-7432-4769-6.
- ^ "Rose, J.D. 2003. A Critique of the paper: "Do fish have nociceptors: Evidence for the evolution of a vertebrate sensory system"" (PDF). Archived from the original (PDF) on 8 June 2011. Retrieved 21 May 2011.
- ^ Rose, James D. (2002). "Do Fish Feel Pain?". Archived from the original on 20 January 2013. Retrieved 27 September 2007.
- ^ Sfakiotakis, M.; Lane, D. M.; Davies, J. B. C. (1999). "Review of Fish Swimming Modes for Aquatic Locomotion" (PDF). IEEE Journal of Oceanic Engineering. 24 (2): 237–252. Bibcode:1999IJOE...24..237S. doi:10.1109/48.757275. S2CID 17226211. Archived from the original (PDF) on 24 December 2013.
- ^ von der Emde, G. (15 May 1999). "Active electrolocation of objects in weakly electric fish". Journal of Experimental Biology. 202 (10): 1205–1215. doi:10.1242/jeb.202.10.1205. PMID 10210662.
- ^ Albert, J. S.; Crampton, W. G. (2006). "Electroreception and Electrogenesis". In Lutz, P. L. (ed.). The Physiology of Fishes. Boca Raton, Florida: CRC Press. pp. 429–470. ISBN 978-0-8493-2022-4.
- ^ Catania, Kenneth C. (20 October 2015). "Electric eels use high-voltage to track fast-moving prey". Nature Communications. 6: 8638. Bibcode:2015NatCo...6.8638C. doi:10.1038/ncomms9638. PMC 4667699. PMID 26485580.
- ^ a b Block, B.A.; Finnerty, JR (1993). "Endothermy in fishes: a phylogenetic analysis of constraints, predispositions, and selection pressures" (PDF). Environmental Biology of Fishes. 40 (3): 283–302. doi:10.1007/BF00002518. S2CID 28644501. Archived from the original on 6 November 2020. Retrieved 1 October 2018.
- ^ Wegner, Nicholas C.; Snodgrass, Owyn E.; Dewar, Heidi; Hyde, John R. (15 May 2015). "Whole-body endothermy in a mesopelagic fish, the opah, Lampris guttatus". Science. 348 (6236): 786–789. Bibcode:2015Sci...348..786W. doi:10.1126/science.aaa8902. ISSN 0036-8075. PMID 25977549. S2CID 17412022.
- ^ Goldman, K.J. (1997). "Regulation of body temperature in the white shark, Carcharodon carcharias". Journal of Comparative Physiology. B Biochemical Systemic and Environmental Physiology. 167 (6): 423–429. doi:10.1007/s003600050092. S2CID 28082417. Archived from the original on 6 April 2012. Retrieved 12 October 2011.
- ^ Carey, F.G.; Lawson, K.D. (February 1973). "Temperature regulation in free-swimming bluefin tuna". Comparative Biochemistry and Physiology A. 44 (2): 375–392. doi:10.1016/0300-9629(73)90490-8. PMID 4145757.
- ^ a b c d e Guimaraes-Cruz, Rodrigo J.; dos Santos, José E.; Santos, Gilmar B. (July–September 2005). "Gonadal structure and gametogenesis of Loricaria lentiginosa Isbrücker (Pisces, Teleostei, Siluriformes)". Rev. Bras. Zool. 22 (3): 556–564. doi:10.1590/S0101-81752005000300005. ISSN 0101-8175.
- ^ Brito, M.F.G.; Bazzoli, N. (2003). "Reproduction of the surubim catfish (Pisces, Pimelodidae) in the São Francisco River, Pirapora Region, Minas Gerais, Brazil". Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 55 (5): 624–633. doi:10.1590/S0102-09352003000500018. ISSN 0102-0935.
- ^ Peter Scott: Livebearing Fishes, p. 13. Tetra Press 1997. ISBN 1-56465-193-2
- ^ a b Meisner, A & Burns, J: Viviparity in the Halfbeak Genera Dermogenys and Nomorhamphus (Teleostei: Hemiramphidae)" Journal of Morphology 234, pp. 295–317, 1997
- ^ a b Pitcher, Tony J. (1986). "12. Functions of Shoaling Behaviour in Teleosts". The Behaviour of Teleost Fishes. Springer. pp. 294–337. doi:10.1007/978-1-4684-8261-4_12. ISBN 978-1-4684-8263-8.
- ^ Helfman, Collette & Facey 1997, p. 375.
- ^ Weinmann, S.R.; Black, A.N.; Richter, M.L.; Itzkowitz, M.; Burger, R.M. (February 2017). "Territorial vocalization in sympatric damselfish: acoustic characteristics and intruder discrimination". Bioacoustics. 27 (1): 87–102. doi:10.1080/09524622.2017.1286263. S2CID 89625932.
- ^ a b Bertucci, F.; Ruppé, L.; Wassenbergh, S.V.; Compère, P.; Parmentier, E. (29 October 2014). "New Insights into the Role of the Pharyngeal Jaw Apparatus in the Sound-Producing Mechanism of Haemulon Flavolineatum (Haemulidae)". Journal of Experimental Biology. 217 (21): 3862–3869. doi:10.1242/jeb.109025. hdl:10067/1197840151162165141. PMID 25355850.
- ^ Colson, D.J.; Patek, S.N.; Brainerd, E.L.; Lewis, S.M. (February 1998). "Sound production during feeding in Hippocampus seahorses (Syngnathidae)". Environmental Biology of Fishes. 51 (2): 221–229. Bibcode:1998EnvBF..51..221C. doi:10.1023/A:1007434714122. S2CID 207648816.
- ^ Oliveira, T.P.R.; Ladich, F.; Abed-Navandi, D.; Souto, A.S.; Rosa, I.L. (26 June 2014). "Sounds produced by the longsnout seahorse: a study of their structure and functions". Journal of Zoology. 294 (2): 114–121. doi:10.1111/jzo.12160.
- ^ a b Fine, L.F.; King, C.B.; Cameron, T.M. (16 October 2009). "Acoustical properties of the swimbladder in the oyster toadfish Opsanus tau". Journal of Experimental Biology. 212 (21): 3542–3552. doi:10.1242/jeb.033423. PMC 2762879. PMID 19837896.
- ^ Fine, M.L.; Waybright, T.D. (15 October 2015). "Grunt variation in the oyster toadfish Opsanus tau:effect of size and sex". PeerJ. 3 (1330): e1330. doi:10.7717/peerj.1330. PMC 4662586. PMID 26623178.
- ^ a b c Ricci, S.W.; Bohnenstiehl, D. R.; Eggleston, D.B.; Kellogg, M.L.; Lyon, R.P. (8 August 2017). "Oyster toadfish (Opsanus tau) boatwhistle call detection and patterns within a large-scale oyster restoration site". PLOS ONE. 12 (8): e0182757. Bibcode:2017PLoSO..1282757R. doi:10.1371/journal.pone.0182757. PMC 5549733. PMID 28792543.
- ^ Skoglund, C.R. (1 August 1961). "Functional analysis of swimbladder muscles engaged in sound productivity of the toadfish". Journal of Cell Biology. 10 (4): 187–200. doi:10.1083/jcb.10.4.187. PMC 2225107. PMID 19866593.
- ^ Parmentier, E.; Tock, J.; Falguière, J.C.; Beauchaud, M. (22 May 2014). "Sound production in Sciaenops ocellatus: Preliminary study for the development of acoustic cues in aquaculture" (PDF). Aquaculture. 432: 204–211. Bibcode:2014Aquac.432..204P. doi:10.1016/j.aquaculture.2014.05.017. Archived (PDF) from the original on 3 June 2021. Retrieved 21 January 2019.
- ^ Helfman, Collette & Facey 1997, pp. 95–96.
- ^ Helfman, Collette & Facey 1997, p. 380.
- ^ Wyman, Richard L.; Ward, Jack A. (1972). "A Cleaning Symbiosis between the Cichlid Fishes Etroplus maculatus and Etroplus suratensis. I. Description and Possible Evolution". Copeia. 1972 (4): 834–838. doi:10.2307/1442742. JSTOR 1442742.
- ^ Zapata, A.G.; Chiba, A.; Vara, A. (1996). "Cells and tissues of the immune system of fish". In Iwama, G. Iwama; Nakanishi, T. (eds.). The Fish Immune System: Organism, Pathogen and Environment. Fish Immunology. New York: Academic Press. pp. 1–55.
- ^ D.P. Anderson. Fish Immunology. (S.F. Snieszko and H.R. Axelrod, eds), Hong Kong: TFH Publications, 1977.
- ^ Chilmonczyk, S. (1992). "The thymus in fish: development and possible function in the immune response". Annual Review of Fish Diseases. 2: 181–200. doi:10.1016/0959-8030(92)90063-4.
- ^ Hansen, J.D.; Zapata, A.G. (1998). "Lymphocyte development in fish and amphibians". Immunological Reviews. 166: 199–220. doi:10.1111/j.1600-065x.1998.tb01264.x. PMID 9914914. S2CID 7965762.
- ^ Küchler, Axel M.; Gjini, Evisa; Peterson-Maduro, Josi; Cancilla, Belinda; Wolburg, Hartwig; Schulte-Merker, Stefan (2006). "Development of the Zebrafish Lymphatic System Requires Vegfc Signaling". Current Biology. 16 (12): 1244–1248. doi:10.1016/j.cub.2006.05.026.
- ^ Flajnik, M. F.; Kasahara, M. (2009). "Origin and evolution of the adaptive immune system: genetic events and selective pressures". Nature Reviews Genetics. 11 (1): 47–59. doi:10.1038/nrg2703. PMC 3805090. PMID 19997068.
- ^ "Table 1: Numbers of threatened species by major groups of organisms (1996–2004)". iucnredlist.org. Archived from the original on 30 June 2006. Retrieved 18 January 2006.
- ^ Sobel, J. (1996). "Gadus morhua". IUCN Red List of Threatened Species. 1996: e.T8784A12931575. doi:10.2305/IUCN.UK.1996.RLTS.T8784A12931575.en. Retrieved 11 November 2021.
- ^ NatureServe (2014). "Cyprinodon diabolis". IUCN Red List of Threatened Species. 2014: e.T6149A15362335. doi:10.2305/IUCN.UK.2014-3.RLTS.T6149A15362335.en. Retrieved 11 November 2021.
- ^ Musick, J.A. (2000). "Latimeria chalumnae". IUCN Red List of Threatened Species. 2000: e.T11375A3274618. doi:10.2305/IUCN.UK.2000.RLTS.T11375A3274618.en. Retrieved 11 November 2021.
- ^ Rigby, C.L.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; et al. (2019). "Carcharodon carcharias". IUCN Red List of Threatened Species. 2019: e.T3855A2878674. Retrieved 19 December 2019.
- ^ Helfman, Collette & Facey 1997, pp. 449–450.
- ^ "Call to halt cod 'over-fishing'". BBC News. 5 January 2007. Archived from the original on 17 January 2007. Retrieved 18 January 2006.
- ^ "Tuna groups tackle overfishing". BBC News. 26 January 2007. Archived from the original on 21 January 2009. Retrieved 18 January 2006.
- ^ Helfman, Collette & Facey 1997, p. 462.
- ^ "UK 'must shield fishing industry'". BBC News. 3 November 2006. Archived from the original on 30 November 2006. Retrieved 18 January 2006.
- ^ "EU fish quota deal hammered out". BBC News. 21 December 2006. Archived from the original on 26 December 2006. Retrieved 18 January 2006.
- ^ "Ocean study predicts the collapse of all seafood fisheries by 2050". phys.org. Archived from the original on 15 March 2007. Retrieved 13 January 2006.
- ^ "Atlantic bluefin tuna could soon be commercially extinct". Archived from the original on 30 April 2007. Retrieved 18 January 2006.
- ^ Helfman, Collette & Facey 1997, p. 463.
- ^ "Threatened and Endangered Species: Pallid Sturgeon Scaphirhynchus Fact Sheet". Archived from the original on 26 November 2005. Retrieved 18 March 2016.
- ^ Atlas of Exotic Fishes in the Mediterranean Sea. 2nd Edition. 2021. (F. Briand Ed.) CIESM Publishers, Paris, Monaco 366 p.[1] Archived 6 December 2022 at the Wayback Machine
- ^ Briand, Frederic; Galil, Bella (November 2002). "Alien marine organisms introduced by ships – An overview". CIESM Workshop Monographs. 20 (5).
- ^ Spinney, Laura (4 August 2005). "The little fish fight back". The Guardian. London. Archived from the original on 9 November 2020. Retrieved 18 January 2006.
- ^ Spalding, Mark (11 July 2013). "Sustainable Ancient Aquaculture". National Geographic. Archived from the original on 18 May 2015. Retrieved 13 August 2015.
- ^ a b Helfman, Gene S. (2007). Fish Conservation: A Guide to Understanding and Restoring Global Aquatic Biodiversity and Fishery Resources. Island Press. p. 11. ISBN 978-1-59726-760-1.
- ^ "EDF Seafood Selector: Fish Choices that are Good for You and the Oceans". Environmental Defense Fund. Retrieved 21 January 2024.
- ^ FAO (2022). The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. Rome: FAO.
- ^ Beard, T. Douglas, ed. (2011). The Angler in the Environment: Social, Economic, Biological, and Ethical Dimensions. Bethesda, MD: American Fisheries Society. p. 365. ISBN 978-1-934874-24-0.
- ^ Hickley, Phil; Tompkins, Helena, eds. (1998). Recreational Fisheries: Social, Economic and Management Aspects. Wiley-Blackwell. p. 328. ISBN 978-0-852-38248-6.
- ^ a b c d e Black, Jeremy; Green, Anthony (1992). Gods, Demons and Symbols of Ancient Mesopotamia: An Illustrated Dictionary. The British Museum Press. pp. 82–83. ISBN 978-0-7141-1705-8. Archived from the original on 20 February 2018.
- ^ a b c d Hyde, Walter Woodburn (2008) [1946]. Paganism to Christianity in the Roman Empire. Eugene, Oregon: Wipf and Stock Publishers. pp. 57–58. ISBN 978-1-60608-349-9. Archived from the original on 17 March 2023. Retrieved 12 December 2020.
- ^ Sherwood, Yvonne (2000), A Biblical Text and Its Afterlives: The Survival of Jonah in Western Culture, Cambridge University Press, pp. 1–8, ISBN 978-0-521-79561-6, archived from the original on 17 March 2023, retrieved 12 December 2020
- ^ a b Coffman, Elesha (8 August 2008). "What is the origin of the Christian fish symbol?". Christianity Today. Archived from the original on 30 January 2016. Retrieved 13 August 2015.
- ^ "Piscis Austrinus". allthesky.com. The Deep Photographic Guide to the Constellations. Archived from the original on 25 November 2015. Retrieved 1 November 2015.
- ^ Zollinger, Sue Anne (3 July 2009). "Piranha – Ferocious Fighter or Scavenging Softie?". A Moment of Science. Indiana Public Media. Archived from the original on 17 October 2015. Retrieved 1 November 2015.
Sources
- Helfman, G.; Collette, B.; Facey, D. (1997). The Diversity of Fishes (1st ed.). Wiley-Blackwell. ISBN 978-0-86542-256-8.
- Nelson, Joseph S. (2006). Fishes of the World (PDF) (4th ed.). John Wiley & Sons. ISBN 978-0-471-75644-6. Archived from the original (PDF) on 5 March 2013. Retrieved 30 April 2013.
Further reading
- Eschmeyer, William N.; Fong, Jon David (2013). "Catalog of Fishes". California Academy of Sciences. Archived from the original on 21 November 2018. Retrieved 28 February 2013.
- Helfman, G.; Collette, B.; Facey, D.; Bowen, B. (2009). The Diversity of Fishes: Biology, Evolution, and Ecology (2nd ed.). Wiley-Blackwell. ISBN 978-1-4051-2494-2. Archived from the original on 26 August 2021. Retrieved 26 January 2010.
- Moyle, Peter B. (1993) Fish: An Enthusiast's Guide Archived 17 March 2023 at the Wayback Machine University of California Press. ISBN 978-0-520-91665-4 – good lay text.
- Moyle, Peter B.; Cech, Joseph J. (2003). Fishes, An Introduction to Ichthyology (5th ed.). Benjamin Cummings. ISBN 978-0-13-100847-2.
- Scales, Helen (2018). Eye of the shoal: A Fishwatcher's Guide to Life, the Ocean and Everything. Bloomsbury Sigma. ISBN 978-1-4729-3684-4.
- Shubin, Neil (2009). Your inner fish: A journey into the 3.5 billion year history of the human body. Vintage Books. ISBN 978-0-307-27745-9. Archived from the original on 17 March 2023. Retrieved 15 December 2015. UCTV interview Archived 14 January 2021 at the Wayback Machine
External links
- ANGFA – Illustrated database of freshwater fishes of Australia and New Guinea
- FishBase online – Comprehensive database with information on over 29,000 fish species
- Fisheries and Illinois Aquaculture Center – Data outlet for fisheries and aquaculture research center in the central US at archive.today (archived 15 December 2012)
- Philippines Fishes – Database with thousands of Philippine Fishes photographed in natural habitat
- The Native Fish Conservancy – Conservation and study of North American freshwater fishes at the Wayback Machine (archived 12 March 2008)
- United Nation – Fisheries and Aquaculture Department: Fish and seafood utilization















