User:organoMetallurgy/Drafts/O-Carborane
| This is not a Wikipedia article: It is an individual user's work-in-progress page, and may be incomplete and/or unreliable. For guidance on developing this draft, see Wikipedia:So you made a userspace draft. Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |

| |
| File:O-carborane.svg | |
| Names | |
|---|---|
| IUPAC name
closo-1,2-dicarbadodecaborane
| |
| Identifiers | |
PubChem CID
|
|
| Properties | |
| Appearance | white powder |
| Melting point | 194–196 °C (381–385 °F; 467–469 K)Doi:10.1021/ja01065a045 In a closed capillary tube |
| Boiling point | readily sublimes |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
non chembox section
[edit]ortho-Carborane, 1,2-C2B10H12, more formally known as closo-dicarbadodecaborane, it was the first carborane discovered and it is the most studied of the carboranes.
Properties
[edit]Synthesis
[edit]o-Carborane is formed by reaction between decaborane(14) and acetylene in the presence of a Lewis base. This method can also be used to prepare some C-substituted derivatives by using other alkynes instead of acetylene.
Uses
[edit]o-Carborane is a precursor to many other carboranes
Materials science
Medicine
Chemistry
[edit]Substitution
[edit]At boron
[edit]o-Carborane undergoes electrophilic substitution reactions at the boron atoms
At carbon
[edit]The C-H bonds are relatively acidic allowing o-carborane to be metalated at the carbon. The metalated derivatives can then be used to prepare C-substituted derivatives of o-carborane. o-Carborane reacts with organolithium reagents to give the 1-lithio and 1,2-dilithio derivatives
Rearrangement
[edit]o-Carborane undergoes thermal rearrangment to meta-carborane at temperatures about 425 °C which further rearranges to para-carborane above 600 °C
Reduction
[edit]Can be reduced to a nido carborane C2B10H12 anion by alkali metals such as lithium and sodium.
Degradation
[edit]Strong bases such as alkoxides can abstact boron to form the nido 1,2-C2B9H12 anion, also known as the 1,2-dicarbollide anion.
Text from the carborane article
[edit]
The most heavily studied carborane is C2B10H12, m. p. 320 °C. It is often prepared from the reaction of acetylene with decaborane. A variation on this method entails the use of dimethyl acetylenedicarboxylate to give C2B10H10(CO2C H3)2, which can be degraded to the C2B10H12.[1]
History
[edit]The 1,2-closo-dicarbadodecaboranes (usually simply called carboranes), were reported simultaneously by groups at Olin Corporation and the Reaction Motors Division of Thiokol Chemical Corporation working under the U.S. Air Force and published in 1963.[2] Heretofore, decaborane derivatives were thought to be thermally unstable and reactive with air and water. These groups demonstrated the unprecedented stability of the 1,2-closo-dodecaborane group, presented a general synthesis, described the transformation of substituents without destroying the carborane cluster, and demonstrated the ortho to meta isomerization.

Isomerization of dicarboranes
[edit]Ortho dicarborane is the kinetic product from the addition of acetylenes to decarborane precursors. Upon heating at 420 °C, the ortho dicarborane rearranges to the meta isomer. Upon further heating to temperatures above 600 °C, one obtains para-carborane. Like arenes, carboranes also undergo electrophilic aromatic substitution.

Thermal rearrangement of o-dicarborane to its meta- and para-isomers (CH vertex indicated with black spheres).
Dicarbollide
[edit]Numerous studies have been made on derivatives of the so-called dicarbollide anion, [B9C2H11]2−. The first metal dicarbollide complex was discovered by M. Frederick Hawthorne and co-workers in 1965.[3] This anion forms sandwich compounds, referred to as bis(dicarbollides), with many metal ions and some exist in otherwise unusual oxidation states. The dianion is a nido cluster prepared by degradation of the parent dicarborane. For many practical syntheses, the monoprotonated dicarbollide salt B9C2H−
12 is isolated as its potassium salt:[4]
- B10C2H12 + 3 CH3OH + KOH → KB9C2H12 + B(OCH3)3 + H2O + H2
The monopotassium salt can then deprotonated with strong base:
- KB10C2H12 + KH → K2B9C2H11 + H2
Bis(dicarbollides) often exhibit properties very different from their metallocene surrogates. For example, Ni-based bis(dicarbollide) cluster can be observed for the rare Ni(IV) oxidation state of nickel. Some notable examples of potential applications of these complexes include catalysis,[5] ion-exchange materials for radioactive waste management, biologically active protease inhibitors, and chemically inert redox shuttles for dye-sensitized solar cells (DSSCs).[6]
Carborynes
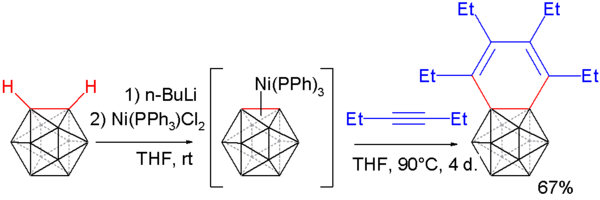
[edit]Carboryne, or 1,2-dehydro-o-carborane, is an unstable derivative of ortho-carborane with the formula B10C2H10. The hydrogen atoms on the C2 unit in the parent o-carborane are missing. The compound resembles and is isolobal with benzyne.[7][8][9] A carboryne compound was first generated in 1990 starting from o-carborane. The hydrogen atoms connected to carbon are removed by n-butyllithium in tetrahydrofuran and the resulting lithium dianion is reacted with bromine at 0 °C to form the bromo monoanion.
Heating the reaction mixture to 35 °C releases carboryne, which can subsequently be trapped with suitable dienes:
such as anthracene (to afford a triptycene-like molecule) and furan in 10 to 25% chemical yield.
Carborynes react with alkynes to benzocarboranes [10][11] in an adaptation of the above described procedure. O-carborane is deprotonated with n-butyllithium as before and then reacted with dichloro-di(triphenylphosphino) nickel to a nickel coordinated carboryne. This compound reacts with 3-hexyne in an alkyne trimerization to the benzocarborane.
Single crystal X-ray diffraction analysis of this compound shows considerable bond length alternation in the benzene ring (164.8 pm to 133.8 pm) ruling out aromaticity.
References
[edit]- ^ Kutal, C. R.; Owen, D. A.; Todd, L. J. (1968). "Closo-1,2-dicarbadodecaborane(12)". Inorganic Syntheses. 11: 19–23. doi:10.1002/9780470132425.ch5. ISBN 978-0-470-13242-5.
- ^ Heying, T. L.; Ager, J. W.; Clark, S. L.; Mangold, D. J.; Goldstein, H. L.; Hillman, M.; Polak, R. J.; Szymanski, J. W. (1963). "A New Series of Organoboranes. I. Carboranes from the Reaction of Decaborane with Acetylenic Compounds". Inorganic Chemistry. 2 (6): 1089–1092. doi:10.1021/ic50010a002.Schroeder, H.; Heying, T. L.; Reiner, J. R. (1963). "A New Series of Organoboranes. II. The Chlorination of 1,2-Dicarbaclosododecaborane(12)". Inorganic Chemistry. 2 (6): 1092–1096. doi:10.1021/ic50010a003.Heying, T. L.; Ager, J. W.; Clark, S. L.; Alexander, R. P.; Papetti, S.; Reid, J. A.; Trotz, S. I. (1963). "A New Series of Organoboranes. III. Some Reactions of 1,2-Dicarbaclosododecaborane(12) and its Derivatives". Inorganic Chemistry. 2 (6): 1097–1105. doi:10.1021/ic50010a004.Papetti, S.; Heying, T. L. (1963). "A New Series of Organoboranes. IV. The Participation of the 1,2-Dicarbaclosododecaborane(12) Nucleus in Some Novel Heteratomic Ring Systems". Inorganic Chemistry. 2 (6): 1105–1107. doi:10.1021/ic50010a005.Fein, M. M.; Bobinski, J.; Mayes, N.; Schwartz, N.; Cohen, M. S. (1963). "Carboranes. I. The Preparation and Chemistry of 1-Isopropenylcarborane and its Derivatives (a New Family of Stable Closoboranes)". Inorganic Chemistry. 2 (6): 1111–1115. doi:10.1021/ic50010a007.Fein, M. M.; Grafstein, D.; Paustian, J. E.; Bobinski, J.; Lichstein, B. M.; Mayes, N.; Schwartz, N. N.; Cohen, M. S. (1963). "Carboranes. II. The Preparation of 1- and 1,2-Substituted Carboranes". Inorganic Chemistry. 2 (6): 1115–1119. doi:10.1021/ic50010a008.Grafstein, D.; Bobinski, J.; Dvorak, J.; Smith, H.; Schwartz, N.; Cohen, M. S.; Fein, M. M. (1963). "Carboranes. III. Reactions of the Carboranes". Inorganic Chemistry. 2 (6): 1120–1125. doi:10.1021/ic50010a009.Grafstein, D.; Bobinski, J.; Dvorak, J.; Paustian, J. E.; Smith, H. F.; Karlan, S.; Vogel, C.; Fein, M. M. (1963). "Carboranes. IV. Chemistry of Bis-(1-carboranylalkyl) Ethers". Inorganic Chemistry. 2 (6): 1125–1128. doi:10.1021/ic50010a010.Grafstein, D.; Dvorak, J. (1963). "Neocarboranes, a New Family of Stable Organoboranes Isomeric with the Carboranes". Inorganic Chemistry. 2 (6): 1128–1133. doi:10.1021/ic50010a011.
- ^ Hawthorne, M. F.; Young, D. C.; Wegner, P. A. (1965). "Carbametallic Boron Hydride Derivatives. I. Apparent Analogs of Ferrocene and Ferricinium Ion". Journal of the American Chemical Society. 87 (8): 1818–1819. doi:10.1021/ja01086a053.
- ^ Plešek, J.; Heřmánek, S.; Štíbr, B. (1983). "Potassium dodecahydro-7,8-dicarba-nido-undecaborate(1-), k[7,8-C2B9H12], intermediates, stock solution, and anhydrous salt". Inorganic Syntheses. 22: 231–234. doi:10.1002/9780470132531.ch53.
- ^ Crowther, D. J.; Baenziger, N. C.; Jordan, R. F. (1991). "Group 4 metal dicarbollide chemistry. Synthesis, structures and reactivity of electrophilic alkyl complexes (Cp*)(C2B9H11)M(R), M = Hf, Zr". Journal of the American Chemical Society. 113 (4): 1455–1457. doi:10.1021/ja00004a080.
- ^ Spokoyny, A. M.; Li, T. C.; Machan, C. W.; Farha, O. K.; She, C.; Stern, C. L.; Marks, T. J.; Hupp, J. T.; Mirkin, C. A. (2010). "Electronic Tuning of Nickel-Based Bis(dicarbollide) Redox Shuttles in Dye-Sensitized Solar Cells". Angewandte Chemie International Edition. 49 (31): 5339–5343. doi:10.1002/anie.201002181. PMID 20586090.
- ^ Gingrich, H. L.; Ghosh, T.; Huang, Q.; Jones, M. (1990). "1,2-Dehydro-o-carborane". Journal of the American Chemical Society. 112 (10): 4082–4083. doi:10.1021/ja00166a080.
- ^ Jemmis, E. D.; Kiran, B. (1997). "Structure and Bonding in B10X2H10 (X = C and Si). The Kinky Surface of 1,2-Dehydro-o-Disilaborane". Journal of the American Chemical Society. 119 (19): 4076–4077. doi:10.1021/ja964385q.
- ^ Kiran, B.; Anoop, A.; Jemmis, E. D. (2002). "Control of Stability through Overlap Matching: closo-Carboranes and closo-Silaboranes". Journal of the American Chemical Society. 124 (16): 4402–4407. doi:10.1021/ja016843n.
- ^ Deng, L.; Chan, H.-S.; Xie, Z. (2006). "Nickel-Mediated Regioselective [2 + 2 + 2] Cycloaddition of Carboryne with Alkynes". Journal of the American Chemical Society. 128 (24): 7728–7729. doi:10.1021/ja061605j. PMID 16771473.
- ^ Jemmis, E. D.; Anoop, A. (2004). "Theoretical Study of the Insertion Reactions of Benzyne- and Carboryne- Ni Complexes" (pdf). Maui High Performance Computing Center Application Briefs. 2004. Air Force Maui Optical & Supercomputing Site (AMOS): 51.[permanent dead link]
- ^ 1935-, Grimes, Russell N.,. Carboranes (Third edition ed.). New York. ISBN 0128019050. OCLC 956655956.
{{cite book}}:|edition=has extra text (help);|last=has numeric name (help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link)