User:R8R/Fluorine
Structural chemistry of the compounds
[edit]
Fluorine's most common oxidation state is −1; it only differs from this value in elemental fluorine, where the atoms are bonded to each other and thus at oxidation state 0, and the very unstable anions F−
2 and F−
3,[2] with intermediate oxidation states, both decomposing at around 40 K. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist.[3] Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding.[4] Fluorine has a rich chemistry including inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds.[note 1]
Inorganic compounds
[edit]Hydrogen fluoride
[edit]Fluorine combines with hydrogen to make a compound (HF) called hydrogen fluoride or, especially in the context of water solutions, hydrofluoric acid. The H-F bond type is one of the few capable of hydrogen bonding (creating extra clustering associations with similar molecules). This influences various peculiar aspects of hydrogen fluoride's properties. In some ways the substance behaves more like water, also very prone to hydrogen bonding, than one of the other hydrogen halides, such as HCl.[5][6][7]
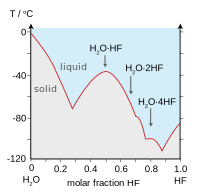
Hydrogen bonding amongst HF molecules gives rise to high viscosity in the liquid phase and lower than expected pressure in the gas phase. Hydrogen fluoride does not boil until 20 °C in contrast to the heavier hydrogen halides which boil between −85 °C and −35 °C (−120–30 °F). HF is fully miscible with water (will dissolve in any proportion), while the other hyrogen halides have large solubility gaps with water. Hydrogen fluoride and water also form several compounds in the solid state, most notably a 1:1 compound that does not melt until −40 °C (−40 °F), which is 44 degrees Celsius (79 degrees Fahrenheit) above the melting point of pure HF.[8]
Unlike other hydrohalic acids, such as hydrochloric acid, hydrogen fluoride is only a weak acid in water solution, with acid dissociation constant (pKa) equal to 3.19.[9] HF's weakness as an aqueous acid is paradoxical considering how polar the HF bond is, much more so than the bond in HCl, HBr, or HI. The explanation for the behavior is complicated, having to do with various cluster-forming tendencies of HF, water, and fluoride ion, as well as thermodynamic issues.[note 2] At great concentrations, a property called homoconjugation is revealed. HF begins to accept fluoride ions, forming the polyatomic ions (such as bifluoride, HF−
2) and protons, thus greatly increasing the acidity of the compound.[11] Hydrofluoric acid is also the strongest of the hydrohalic acids in acetic acid and similar solvents.[12] Its hidden acidity potential is also revealed by the fact it protonates acids like hydrochloric, sulfuric, or nitric.[13] Despite its weakness, hydrofluoric acid is very corrosive, even attacking glass (hydrated only).[11]
Dry hydrogen fluoride dissolves low-valent metal fluorides readily. Several molecular fluorides also dissolve in HF. Many proteins and carbohydrates can be dissolved in dry HF and can be recovered from it. Most non-fluoride inorganic chemicals react with HF rather than dissolving.[14]
Metal fluorides
[edit]Metal fluorides have similarities with other metal halides but are more ionic. In many respects, metal fluorides differ from other metal halides (chlorides, bromides, iodides), very similar to each other. Instead, fluorides are more similar to oxides, often having similar bonding and crystal structures.[15]
The metal fluorides show broad trends based on the charge of the metal. Metals in an oxidation state of +3 or lower tend to form ionic, refractory fluorides. Metals charged +5 or higher tend to form covalently bonded fluorides, polymers or discrete molecules, and are more volatile. (The tetrafluorides are a transition zone.) The bonding variations mean that metal fluorides can be solids,[16] liquids,[17] or gases[18] at room temperature.
The solubility of fluorides varies greatly but tends to decrease as the charge on the metal ion increases. Dissolved fluorides produce basic solutions. (F- is a weak base because HF is a weak acid.)[19] Like hydroxides, fluorides may be viewed as basic, amphoteric, and acidic, with the acidity property generally increasing with the oxidation state of the metal; a fluoride is a way weaker base than a hydroxide, though.
| The fluorides of transition metal elements 25–29 | ||||

|

|

|

|

|
| Manganese difluoride | Iron trifluoride | Cobalt difluoride | Nickel difluoride | Copper difluoride |
Low oxidation state metal fluorides
[edit]
The alkali metals form monofluorides. All are soluble and have the sodium chloride (rock salt) structure,[20] which is also adopted by some alkaline earth oxides such as CaO.[21] Because the fluoride anion is highly basic, many alkali metal fluorides form bifluorides with the formula MHF2. They also give off the fluoride easily when reacting with an acid. Among other monofluorides, only silver(I)[22] and thallium(I)[23] fluorides are well-characterized. Both are very soluble, unlike the other halides of those metals. Another silver fluoride is from this point a "half-fluoride" (a subfluoride, compound containing less than normal saturation with fluorine), formulated as Ag
2F. This compound has been described as having F- and unusual Ag½+ centers.[24]
Unlike the monofluorides, the difluorides may be either soluble or insoluble. Several transition metal difluorides, such as those of copper(II) and nickel(II), are soluble.[22] The alkaline earth metals form difluorides that are insoluble.[22] In contrast, the alkaline earth chlorides are readily soluble.[22] Many of the difluorides adopt the fluorite structure, named after calcium fluoride (and also adopted by several metal dioxides such as CeO2, UO2, ThO2, etc.), which surrounds each metal cation with 8 fluorides. Some difluorides adopt the rutile structure, named after a form of titanium dioxide and adopted by several other metal dioxides also. The structure is tetragonal and puts metal atoms in octahedral coordination.
Beryllium difluoride is different than the other difluorides, just is beryllium different than other alkaline earth metals. In general, beryllium has a tendency to bond covalently, much more so than the other alkaline earths and its fluoride is partially covalent (although still more ionic than its other halides). BeF2 has many similarities to SiO2 (quartz) a mostly covalently bonded network solid. BeF2 has tetrahedrally coordinated metal and forms glasses (is difficult to crystallize). When crystalline, beryllium fluoride has the same room temperature crystal structure as quartz and shares many higher temperatures structures also. It is very soluble in water,[22] unlike the other alkaline earths. (Although they are strongly ionic, they do not dissolve because of the especially strong lattice energy of the fluorite structure.) However, BeF2 has much lower electrical conductivity when in solution or when molten than would be expected if it were fully ionic.[25][26][27][28]
| Order and disorder in difluorides | |

|

|
| The fluorite structure | Beryllium fluoride glass |
Many metals form trifluorides, such as iron, bismuth, the rare earth elements, and the metals in the aluminium column of the periodic table. The trifluorides of many rare earths as well as bismuth have the YF3 structure. Trifluorides of plutonium, samarium (at high temperature), and lanthanum adopt the LaF3 structure. Iron and gallium trifluorides have the FeF3 structure which is similar to rhenium trioxide.
At this point, the ionicity of the fluorides begins to reduce, and acidity begins to grow, even though the compounds are still ionic solids, mainly weakly basic. In many cases, while the trifluorides are still ionic, other trihalides may be volatile (for example, aluminium[29] or gold[30]). No trifluoride is soluble in water, but several are soluble in other solvents.[31]
The tetrafluorides show a mixture of ionic and covalent bonding: Zirconium, hafnium, plus many of the actinides form square-antiprism ionic,[32][33] high-melting[34] tetrafluorides.
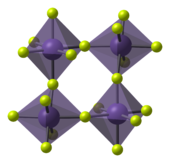
Titanium, tin and vanadium[36] tetrafluorides are polymeric, with melting points below 400 °C (vanadium's disproportionates at 100–120 °C to the trifluoride and the pentafluoride[37]). Many transition metals tetrafluorides show unusual structures intermediate between ionic and monomeric covalent. They have low melting points or are of low stability (manganese tetrafluoride decomposes even at room temperature[38]).
Germanium tetrafluoride forms tetrahedral molecules. It is a gas at room temperature.[39]
High oxidation state metal fluorides
[edit]Metal penta- and higher fluorides are all covalently bonded and volatile. This behavior contrasts with the corresponding oxides. Oxygen is a weaker oxidant and inherently more likely to form covalent bonds, but it only forms molecules with six metals (manganese heptoxide, technetium heptoxide, ruthenium tetroxide, osmium tetroxide, plutonium tetroxide,[40] and iridium tetroxide[41]). Fluorine forms molecules with fourteen metals because its small size and single charge as an ion allows surrounding metal atoms with more fluorines than oxygen can.
Bismuth highest fluoride is a volatile penta species that is a powerful fluorinating agent. In the solid state, it is polymeric, consisting of linear chains of octahedra, sharing axial fluorides. Pentavalent bismuth behaves as an acid and forms hexafluorobismuthate, [BiF6]-, upon reaction with a fluoride donor, either strong (such as NaF[42][43]) or not (such as XeF4[44]).
Many metals that form hexafluorides also can form pentafluorides. For instance, uranium, which has a well-known hexafluoride, also forms two different pentafluoride structures. The room-temperature (alpha) form has the same linear chain structure as bismuth pentafluoride. As a molecular (gas) species, UF5 has a square pyramidal structure. Vanadium pentafluoride is said to share this structure, but is reported to be nonvolatile.[45]
| The structure of bismuth pentafluoride | |
  |
  |
| Structure of a (XF5)n chain; X=V,Bi,U. | Packing of chains |

The metals that make well-characterized hexafluorides include nine metals in the center of the periodic table (molybdenum, technetium, ruthenium, rhodium, tungsten, rhenium, osmium, iridium, and platinum) along with elements 92–94: uranium, neptunium, and plutonium. At room temperature, tungsten hexafluoride is a gas. Molybdenum hexafluoride and rhenium hexafluoride are liquids. The rest are volatile solids.
Metal hexafluorides are oxidants because of their tendency to release fluorines: for example, platinum hexafluoride was the first compound to oxidize molecular oxygen[46] and xenon.[47] Polonium also forms a hexafluoride, but it is understudied.[48]
Rhenium is the only metal known to bond with seven fluorides in charge-neutral metal compound, which is the charged-ligands number record.[49] Rhenium heptafluoride adopts a pentagonal bipyramid molecular geometry. Calculations shows that the currently unknown but perhaps possible iridium heptafluoride[50] (report of synthesis is being prepared[41]), technetium heptafluoride,[51] and osmium heptafluoride[52] will also have this structure.

Osmium octafluoride was first reported in 1913, but in 1958 that compound was shown to be actually osmium hexafluoride.[53] A 1993 theoretical study predicted very weak bonds in osmium octafluoride and said that it would be difficult to ever detect experimentally. The study predicted that, if made, OsF8 would have Os–F bonds of two different lengths.[54]
Nonmetal fluorides
[edit]The nonmetal binary fluorides are volatile compounds. They show a great difference between period 2 and other fluorides. For instance, period 2 elements elements fluorides never exceed the octet in their atoms. (Boron is an exception due to its specific position in the periodic table.) Lower-period elements, however, may form hypervalent molecules, such as phosphorus pentafluoride or sulfur hexafluoride.[55] The reactivity of such species varies greatly: sulfur hexafluoride is inert, while chlorine trifluoride is extremely reactive. But there are some tendencies and trends based on periodic table columns.
Boron trifluoride is a planar molecule. It has only six electrons around the central boron atom (and thus an incomplete octet), but it readily accepts a Lewis base, forming adducts with lone-pair-containing molecules or ions such as ammonia or another fluoride ion which can donate two more electrons to complete the octet.[56] Boron monofluoride is an unstable gas that has been isolated at low temperatures. It is a "subfluoride" (compound containing less than normal saturation with fluorine) and is prone to polymerizing. It is stable as a ligand in some metal complexes. The bond order has been described alternately as 3 (a triple bond)[3] and as 1.4 (intermediate between a single and double bond).[57] The molecule is unusual as on it, fluorine is the one to hold the positive charge, despite the electronegativities (although the oxidation state remains -1).

Silicon tetrafluoride, similar to carbon tetrafluoride and germanium tetrafluoride, adopts a molecular tetrahedral structure.[58] SiF4 is stable against heating or electric spark, but reacts with water (even moist air), metals, and alkalies, thus demonstrating weak acidic character.[59] Reactions with organomagnesium compounds, alcohols, amines, and ammonia yield adduction compounds.[59] Fluorosilicic acid, a derivative of SiF4 is a strong acid in aqueous solution (the anhydrous form does not exist).[60]
Among the pnictogens (nitrogen's periodic table column) trifluorides, reactivity and acidity of fluorides increases down the group: NF3 is stable against hydrolysis,[61] PF3 hydrolyzes very slowly in moist air,[62] while AsF3 completely hydrolyzes.[61] SbF3 hydrolyzes only partially due to the increasing ionic character of the bond to fluorine. The compounds are weak Lewis bases, with NF3 again being an exception.[61] The pentafluorides of phosphorus[62] and arsenic[63] are much more reactive than their trifluorides; antimony pentafluoride is such a strong acid that it holds the title of the strongest Lewis acid.[63]
Nitrogen is not known to form a pentafluoride, although the tetrafluoroammonium cation (NF+
4) features nitrogen in the formal oxidation state of +5.[64] Nitrogen and fluorine also combine to make dinitrogen difluoride. Both cis and trans forms of the molecule exist. (The nitrogens are double bonded to each other.) A related N2F+ cation is also known. They two, among others, also form a simple molecule NF, isoelectronic with O2. The ground state of the molecule has a very small negative charges on fluorine, and both higher-energy states shift the positive charge on fluorine again.
The chalcogens (oxygen's periodic table column) mainly form tetrafluorides and hexafluorides. The tetafluorides are thermally unstable and hydrolyze, and are also ready to use their lone pair to form adducts to other (acidic) fluorides. Sulfur and selenium tetrafluorides molecules are trigonal pyramids, while TeF4 is a polymer.[65] The hexafluorides are the result of direct fluorination of the elements (compare: other hexahalides of the elements do even not exist). They, like the previous group's highest fluorides, increase in reactivity with atomic number: SF6 is extremely inert, SeF6 is less noble (for example, reacts with ammonia at 200 °C (400 °F), and TeF6 easily hydrolyzes to give an oxoacid.[65]
Oxygen is (similarly to nitrogen) out of line: Its highest fluoride is oxygen difluoride,[65] and fluorine again can (however, for this element only theoretically as of 2012) oxidize it to a uniquely high oxidation state of +4 in a fluorocation, OF+
3.[66]
The well-characterized heavier halogens (chlorine, bromine, and iodine) all form mono-, tri-, and pentafluorides: XF, XF3, and XF5. Of the neutral +7 species, only iodine heptafluoride is known.[67] While chlorine and bromine heptafluorides are not known, the corresponding cations ClF+
6 and BrF+
6, extremely strong oxidizers, are.[68] Astatine is not well-studied, and although there is a report of a non-volatile astatine monofluoride,[69] its existence is debated.[70]

Many of the halogen fluorides are powerful fluorinators. Chlorine trifluoride is particularly noteworthy—readily fluorinating asbestos and refractory oxides—and may be even more reactive than chlorine pentafluoride. Used industrially, ClF3 requires special precautions similar to those for fluorine gas because of its corrosiveness and hazards to humans.[71][72] The structure of the molecule is very close to a T.
Superacids
[edit]Several important inorganic acids contain fluorine. They are generally very strong because of the high electronegativity of fluorine. One such acid, fluoroantimonic acid (HSbF6), is the strongest charge-neutral acid known.[73] The dispersion of the charge on the anion affects the acidity of the solvated proton (in form of H
2F+
): The compound has an extremely low pKa of −28 and is 10 quadrillion (1016) times stronger than pure sulfuric acid.[73] Fluoroantimonic acid is so strong that it protonates otherwise inert compounds like hydrocarbons. Hungarian-American chemist George Olah received the 1994 Nobel Prize in chemistry for investigating such reactions.[74]
Noble gas compounds
[edit]The noble gases are generally non-reactive because they have fully filled electronic shells, which are extremely stable. Until the 1960s, no chemical bond with a noble gas was known. In 1962, Neil Bartlett used fluorine-containing platinum hexafluoride to react with xenon. He called the compound he prepared xenon hexafluoroplatinate, but since then the product has been revealed to be mixture of different chemicals. Bartlett probably synthesized a mixture of monofluoroxenyl(II) hexafluoroplatinate, [XeF]+[PtF6]–, monofluoroxenyl(II) undecafluorodiplatinate, [XeF]+[Pt2F11]–, and trifluorodixenyl(II) hexafluoroplatinate, [Xe2F3]+[PtF6]–.[75] Bartlett's fluorination of xenon has been called one of the ten most beautiful experiments in the history of chemistry.[76] Later in 1962, xenon was reacted directly with fluorine to form the di- and tetra- fluorides. Since then, chemists have made extensive efforts to form other noble gas fluorides.

Having started the noble gases–fluorine compounds class, xenon is also the noble gas to have the most well-known such compounds. Its binary compounds include xenon difluoride, xenon tetrafluoride, and xenon hexafluoride, as well as their derivatives.[77] Xenon forms several oxyfluorides, such as xenon oxydifluoride, XeOF2, by reaction of xenon tetrafluoride with water.[78] Its upper neighbor, krypton, is the only other one to form a well-established compound: krypton difluoride. Krypton tetrafluoride was reported in 1963,[79] but was subsequently shown to be a mistaken identification;[80] the compound seems to be very hard to synthesize now (although even the hexafluoride may exist).[80] A krypton monofluoride radical[81] (used in the krypton fluoride laser) and cation[82] have been observed at low temperature.
In strong accordance with the periodic trends, radon is notably more reactive to oxidizers, including fluorine. It has been shown to readily react with fluorine to form a solid compound, which is generally thought to be radon difluoride. The exact composition is uncertain as the compound is prone to decomposition. Calculations indicate that radon difluoride may be ionic, unlike all other binary noble gas fluorides.[69]
The lighter noble gases (helium through argon) do not form stable binary fluorides. Argon forms no binary fluoride, but reacts in extreme conditions with hydrogen fluoride to form argon fluorohydride; it is the only "stable" (i.e. not decaying chemically over time under certain conditions) argon compound. Argon also forms a short-lived, metastable binary argon monofluoride, ArF•, which is used in the argon fluoride laser.[83] Helium and neon do not form any stable chemical compounds at all. Helium forms a temporary helium fluorohydride that is stable for a few nanoseconds.[84][85] Neon, the least reactive element,[note 3] forms a metastable chemical compound, neon monofluoride, NeF•.[88]
Ununoctium, the last currently known group 18 element, is predicted to form ununoctium difluoride, UuoF
2, and ununoctium tetrafluoride, UuoF
4, which is likely to have the tetrahedral molecular geometry.[89] However, only a few atoms of ununoctium have been synthesized,[90] and its chemical properties have not been examined.
Highest oxidation states: fluorine versus oxygen
[edit]| Ruthenium's highest fluoride and oxide | |

|

|
| Ruthenium hexafluoride: Six fluorines fit around the ruthenium but only make a +6 oxidation state. | Ruthenium tetroxide: Four oxygens fit around the ruthenium, making a +8 oxidation state. |
Elements frequently have their highest oxidation state in the form of a binary fluoride. Several elements show their highest oxidation state only in a few compounds, one of which is the fluoride; and some elements' highest known oxidation state is seen exclusively in a fluoride.
Fluorine was the first element able to oxidize a group 12 element to an oxidation state above +2, making the element's d-electrons participate in bonding.[91] Mercury(IV) fluoride was produced by this reaction, the first ever mercury(IV) compound; its discovery has heated the debate over whether mercury, cadmium, and zinc are transition metals.[92] The other two such states are cobalt(V)[51] and krypton(II).[93] Silver(V) may be possible in a fluorine species, which would beat the oxygen species (silver(III) is the highscore for the both), the same is true for palladium(VI) (highscores are palladium(IV)) and iridium(VII) (highscores are iridium(VI)).[51] Technetium(VII) is known for the oxide and not the fluoride, but the latter is also possible; osmium(VIII) is analogous from this point.[51] It is possible that element 113, ununtrium, will be the first boron group element to form a species in the +5 oxidation state, the fluorine-based hexafluoroununtrate(V), UutF−
6;[94] no other ununtrium(V) species is expected. In some cases the oxides can go significantly further, though.[51]
For groups 1–5, 10, 13–16, the highest oxidation states of oxides and fluorides are always equal. Differences are only seen in chromium, groups 7–9, copper, mercury, and the noble gases. Fluorination allows elements to achieve relatively low[note 4] oxidation states that are, however, hard to achieve. For example, no binary oxide is known for krypton, but krypton difluoride is well-studied.[93] At the same time, very high oxidation states are known for oxygen-based species only. For the previously mentioned volatile oxides, there are no corresponding hepta- or octafluorides. (For example, ruthenium octafluoride is unlikely to be ever synthesized,[51] while ruthenium tetroxide has even found an industrial use.[95]) The main problem that prevents fluorine from forming the highest states in covalent hepta- and octafluorides is that it is hard to attach such a large number of ligands around a single atom; the number of ligands is halved in analogous oxides.[96][note 5] However, octafluoride anions, such as the octafluororhenate (ReF−
8), octafluorozirconate (ZrF4−
8), and octafluoroxenate (XeF2−
8) anions are well-known.
Organic compounds
[edit]This section needs additional citations for verification. (May 2012) |
Organofluorine compounds are defined by having a carbon–fluorine chemical bond. This bond is the strongest bond in organic chemistry and is very stable.[97] Fluorine replaces hydrogen in hydrocarbons even at room temperature. After the reaction, the molecular size is not changed significantly. Organofluorine compounds are synthesized via both direct reaction with fluorine gas, which can be dangerously reactive, or reaction with fluorinating reagents such as cobalt trifluoride.[98] The range of organofluorine compounds is diverse, reflecting the inherent complexity of organic chemistry. A vast number of small molecules exist with varying amounts of fluorine substitution, as well as many polymers—research into particular areas is driven by the commercial value of applications.[98]

Small molecules
[edit]
Organic compounds with all C-H bonds changed to C-F are called fluorocarbons or perfluorocarbons (the "per" prefix signifying presence of as much fluorine as chemically possible). The very different molecular interaction potentials of perfluorocarbons causes these substances to be extremely insoluble in water and also not miscible with hydrocarbons, thus forming three layers of liquid (perfluorocarbon most dense) if liquid perfluorocarbons are mixed with liquid oil and water.
The perfluoro derivatives of alkanes (hydrocarbons with single bonds only) have higher density and often higher melting and boiling points, and are also more thermally and chemically stable than their hydrocarbon analogs. However, the very small intermolecular interaction energies typical of perfluorinated molecules causes perfluoroalkanes to have much lower melting and boiling points than hydrocarbons of similar molecular weight (sometimes even lower than the hydrocarbon analogs).
In contrast, the perfluorination of hydrocarbons that contain double bonds (alkenes) or triple bonds (alkynes) gives rise to very easily attacked molecules. Difluoroacetylene, which is explosive even at low temperatures, is a notable example.
The Fowler process produces commercial perfluoroalkanes. Hydrocarbon feedstocks are passed over a bed of cobalt trifluoride, which loses fluorine and is reduced to cobalt difluoride. Fluorine reacts with the hydrocarbon producing perfluorocarbon and HF. The cobalt bed is periodically refluorinated to the trifluoride, in a separate step, by reaction with concentrated HF.
Partially fluorinated alkanes exist as well, the hydrofluorocarbons (HFCs). Substituting other halogens in combination with fluorine gives rise to chlorofluorocarbons (CFCs) or bromofluorocarbons (BFCs) and the like. (Or if some hydrogen is retained, HCFCs and the like.) Properties depend on the number and identity of the halogen atoms. In general, the boiling points are even more elevated by combination of halogen atoms because the varying size and charge of different halogens allows more intermolecular attractions.[99] As with fluorocarbons, chlorofluorocarbons and bromofluorocarbons are not flammable: they do not have carbon–hydrogen bonds to react and released halides quench flames.[99]
Perfluorinated compounds (as opposed to perfluorocarbons) is the term used for molecules that would be perfluorocarbons—only carbon and fluorine atoms—except for having an extra functional group. The perfluoro parts of the molecule tend to be hydrophobic and slippery. The functional group allows reactions, attachment to solids, solubility, etc. Electrochemical fluorination (ECF), essentially electrolysis in HF, is the commercial production method.
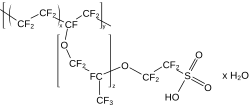
The fluorosurfactants are notable perfluorinated compounds. They have a medium length straight chain of perfluoroalkane terminated by an acid group. This type of arrangement, like a fatty acid, gives rise to a combination of properties. For instance perfluorooctanesulfonic acid (PFOS, formerly the active component in brand "Scotchgard") has eight fluorinated carbons in a row ending with a sulfonic acid group.
For fluorinated organic acids, the large inductive effect of the trifluoromethyl group results in high acid strengths, which may be comparable to mineral acids. In these compounds, the anion's affinity for the acid proton is decreased by the acid's fluorine content, which increases its affinity for the extra electron left when the acidic proton leaves. For example, acetic acid is a weak acid, with pKa equal to 4.76, while its fluorinated derivative, trifluoroacetic acid has pKa of −0.23, giving it 33,000 times greater acid strength.[100]
Polymers
[edit]Fluoropolymers are organic polymers ("plastics") containing fluorine. Polytetrafluoroethylene (PTFE, DuPont brand Teflon) is a simple linear chain polymer with the repeating structural unit: –CF2–. PTFE has a backbone of carbons single bonded in a long chain, with all side bonds to fluorines. It contains no hydrogens and can be thought of as the perfluoro analog of polyethylene (structural unit: –CH2–). PTFE has high chemical and thermal stability, as expected for a perfluorocarbon, much stronger than polyethylene. The non-stick nature of PTFE results from the repulsion of highly charged fluorine atoms in polymeric chains. Its resistance to van der Waals forces makes PTFE the only known surface to which a gecko cannot stick.[101]
Several other fluoropolymers exist that are more complicated structurally than PTFE. FEP (fluorinated ethylene propylene) and PFA (perfluoroalkoxy) are similar to PTFE in not containing any hydrogens and contain a PTFE backbone. FEP has branching chains of fluoroalkane. PFA also has branched fluoralkane but with an ether (oxygen) link. Three fluoropolymers that contain hydrogen are polyvinylidene fluoride (PVDF, structural unit: –CF2CH2–), polyvinyl fluoride (PVF, structural unit: –CH2CHF–), and ethylene tetrafluoroethylene (ETFE, structural unit: –CF2CF2CH2CH2–).
Nafion is a complicated polymer, structurally. It has a PTFE-like backbone, but also contains side chains of perfluoro ether that end in sulfonic acid (SO3H) groups. It has similar chemical stability as PTFE, but because of the acid side chains, is also an ionic conductor, the first ionomer.
References
[edit]- ^ Calderazzo, Fausto (March 2010). "Halide-bridged polymers of divalent metals with donor ligands – structures and properties". Coordination Chemistry Reviews. 254 (5–6): 537–554. doi:10.1016/j.ccr.2009.08.007.
{{cite journal}}: CS1 maint: date and year (link) - ^ Wiberg, Wiberg & Holleman 2001, p. 422.
- ^ a b Allan McQuarrie, Donald; Simon, John Douglas (1997). Physical Chemistry: A Molecular Approach. University Science Books. p. 365. ISBN 0-935702-99-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Smart, Bruce E.; Tatlow, J. C. (1994). Organofluorine Chemistry: Principles and Commercial Application. Springer. p. 515. ISBN 9780306646108.
{{cite book}}: Check|isbn=value: checksum (help)CS1 maint: multiple names: authors list (link) - ^ Pauling, Linus A. (1960). The nature of the chemical bond and the structure of molecules and crystals: an introduction to modern structural chemistry. Ithaca, New York: Cornell University Press. pp. 454–464. ISBN 978-0-8014-0333-0.
- ^ Atkins, Peter (2008). Chemical Principles: The Quest for Insight. W. H. Freeman & Co. pp. 184–185. ISBN 978-1-4292-0965-6.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Emsley, John (30 August 1981). "The hidden strength of hydrogen". New Scientist. 91 (1264): 291–292.
- ^ Greenwood & Earnshaw 1998, pp. 812–816.
- ^ Wiberg, Wiberg & Holleman 2001, p. 425.
- ^ Clark, Jim. "The Acidity of the Hydrogen Halides". Retrieved 4 September 2011.
- ^ a b Chambers, C.; Holliday, A. K. (1975). Modern inorganic chemistry (an intermediate text) (PDF). The Butterworth Group. pp. 328–329.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Hannan, Henry J. (1975). Technician's Formulation Handbook for Industrial and Household Cleaning Products. Lulu.com. p. 31. ISBN 9780615156019.
- ^ Hannan, Henry J. (2010). Course in Chemistry for IIT-JEE 2011. Tata McGraw Hill Education Private Limited. pp. 15–22. ISBN 9780070703360.
- ^ Greenwood & Earnshaw 1998, pp. 816–819.
- ^ Greenwood & Earnshaw 1998, p. 819.
- ^ Lide 2004, p. 4-76.
- ^ Lide 2004, p. 4-71.
- ^ Lide 2004, p. 4-92.
- ^ Oxtoby, David W.; Gillis, H. Pat; Campion, Alan (2012). Principle of Modern Chemistry. Cengage Learning. p. 693. ISBN 9780840049315.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Aigueperse et al. 2005, "Fluorine Compounds, Inorganic," pp. 25–27.
- ^ Arai, Toshihiro (1999). Mesoscopic Materials and Clusters: Their Physical and Chemical Properties. Springer. p. 267. ISBN 9783540648840.
- ^ a b c d e Storer, Frank Humphreys (1864). First Outlines of a Dictionary of Solubilities of Chemical Substances. Cambridge. pp. 278–80. ISBN 978-1-176-62256-2.
- ^ Remy, Heinrich (1956). Treatise on Inorganic Chemistry: Introduction and main groups of the periodic table. Elsevier Publishing Company. p. 383.
- ^ Wiberg, Wiberg & Holleman 2001, p. 1268.
- ^ Emeléus & Sharpe 1983, pp. 256–277.
- ^ Walsh, Kenneth A. (January 2009). Beryllium chemistry and processing. ASM International. pp. 99–102, 118–119. ISBN 978-0-87170-721-5.
- ^ Mackay, Mackay & Henderson 2002, p. 243–244.
- ^ Hertz, Raymond K. (1987). "General analytical chemistry of beryllium". In Coyle, Francis T. (ed.). Chemical analysis of metals: a symposium. ASTM. pp. 74–75. ISBN 978-0-8031-0942-1.
- ^ Wiberg, Wiberg & Holleman 2001, p. 1047.
- ^ Wiberg, Wiberg & Holleman 2001, p. 1286.
- ^ Sobolev, Boris Petrovich (2001). The Rare Earth Trifluorides: Introduction to materials science of multicomponent metal fluoride crystals. Institut d'Estudis Catalans. p. 51. ISBN 84-7283-610-X.
- ^ Kern, S.; Hayward, J.; Roberts, S.; Richardson, J. W.; Rotella, F. J.; Soderholm, L.; Cort, B.; Tinkle, M.; West, M.; Hoisington, D.; Lander, G. A. (1994). "Temperature Variation of the Structural Parameters in Actinide Tetrafluorides". The Journal of Chemical Physics. 101 (11): 9333–9337. Bibcode:1994JChPh.101.9333K. doi:10.1063/1.467963.
- ^ Brown, Paul L.; Mompean, Federico J.; Perrone, Jane; Illemassène, Myriam (2005). Chemical Thermodynamics of Zirconium. Gulf Professional Publishing. p. 144. ISBN 0-444-51803-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Lide 2004, pp. 4–60, -76, -92, -96.
- ^ Nakajimȧ, Tsuyoshi; Žemva, Boris; Tressaud, Alain (2009). Advanced Inorganic Fluorides: Synthesis, Characterization, and Applications (1st ed.). Elsevier. p. 111. ISBN 978-0444720023.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Becker, S.; Muller, B. G. (1990). "Vanadium Tetrafluoride". Angewandte Chemie International Edition. 29 (4): 406. doi:10.1002/anie.199004061.
{{cite journal}}: More than one of|number=and|issue=specified (help) - ^ "Vanadium tetrafluoride". Retrieved 2012-05-13.
- ^ Brown, David; Canterford, J. H.; Colton, Ray (2009). Halides of the Transition Elements: Halides of the first row transition metals, by R. Colton and J. H. Canterford. Wiley. p. 213. Retrieved 2012-06-06.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Yaws & Braker 2001, p. 397.
- ^ Kiselev, Yu. M.; Nikonov, M. V.; Tananaev, I. G.; Myasoedov, B. F. (2009). "On the Existence of Plutonium Tetroxide". Doklady Akademii Nauk. 425 (5). Pleiades Publishing, Ltd.: 634–637. doi:10.1134/S0012501609040022. ISSN 0012-5016. S2CID 96099149. Retrieved 2012-05-26.
- ^ a b Technische Universität Berlin (2012). "Prediction of new compounds and new oxidation states". Retrieved 2012-05-24.
- ^ Breunig, Hans Joachim. "Bismuth compounds". Kirk-Othmer Encyclopedia of Chemical Technology Volume 4. John Wiley & Sons. p. 22.
- ^ Wiberg, Wiberg & Holleman 2001, p. 770.
- ^ Suzuki, Hitomi; Matano, Yoshihiro (2001). Organobismuth chemistry. Elsevier. p. 8. ISBN 0-444-20528-4.
- ^ Emeléus & Sharpe 1983, p. 105.
- ^ Bartlett, Neil; Lohmann, D. H. (1962). "Dioxygenyl hexafluoroplatinate (V), O2+[PtF6]−". Proceedings of the Chemical Society (3). Chemical Society: 115. doi:10.1039/PS9620000097.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bartlett, Neil (1962). "Xenon hexafluoroplatinate (V) Xe+[PtF6]−". Proceedings of the Chemical Society (6). Chemical Society: 218. doi:10.1039/PS9620000197.
- ^ Wiberg, Wiberg & Holleman 2001, p. 594.
- ^ Vogt, T.; Fitch, A. N.; Cockcroft, J. K. (1994). "Crystal and molecular structures of rhenium heptafluoride". Science. 263 (5151): 1265–67. Bibcode:1994Sci...263.1265V. doi:10.1126/science.263.5151.1265. PMID 17817431. S2CID 20013073.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bayerische Julius-Maximilians-Universität Würzburg 2006, p. 93.
- ^ a b c d e f Riedel, S.; Kaupp, M. (2009). "The highest oxidation states of the transition metal elements". Coordination Chemistry Reviews. 253 (5–6): 606–624. doi:10.1016/j.ccr.2008.07.014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bayerische Julius-Maximilians-Universität Würzburg 2006, p. 102.
- ^ Weinstock, Bernard; Malm, John G. (September 1958). "Osmium Hexafluoride and its Identity with the Previously Reported Octafluoride". Journal of the American Chemical Society. 80 (17): 4466–4468. doi:10.1021/ja01550a007.
{{cite journal}}: CS1 maint: date and year (link) - ^ a b Veldkamp, Achim; Frenking, Gernot (June 1993). "Quantum-Mechanical ab initio Investigation of the Transition-Metal Compounds OsO4, OsO3F2, OsO2F4, OsOF6, and OsF8". Chemische Berichte. 126 (6): 1325–1330. doi:10.1002/cber.19931260609.
{{cite journal}}: CS1 maint: date and year (link) - ^ Noury, Stephane; Silvi, Bernard; Gillespie, Ronald J. (2002). "Chemical Bonding in Hypervalent Molecules: Is the Octet Rule Relevant?" (PDF). Inorganic Chemistry. 41 (8): 2164–72. doi:10.1021/ic011003v. PMID 11952370. Retrieved 2012-05-23.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Greenwood & Earnshaw 1998, pp. 198–199.
- ^ Martinie, R. J.; Bultema, J. J.; van der Wal, M. N.; Burkhart, B. J.; van der Griend, D. A.; de Kock, R. L. (2011). "Bond Order and Chemical Properties of BF, CO, and N2" (PDF). Journal of Chemical Education. 88 (8): 1094–1097. doi:10.1021/ed100758t.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ellis, Bryan David (2001). Scientific Essentialism. Campbridge Universuty Press. p. 69. ISBN 0521800943.
- ^ a b Aigueperse et al. 2005, "Fluorine Compounds, Inorganic," p. 28.
- ^ Aigueperse et al. 2005, "Fluorine Compounds, Inorganic," p. 30.
- ^ a b c Raghavan, P. S. (1998). Concepts and Problems in Inorganic Chemistry. Discovery Publishing House. pp. 164–165. ISBN 978817141418.
{{cite book}}: Check|isbn=value: length (help) - ^ a b Aigueperse et al. 2005, "Fluorine Compounds, Inorganic," p. 37.
- ^ a b Norman, Nicholas C. (1998). Chemistry of Arsenic, Antimony and Bismuth. Springer. p. 97. ISBN 075140389X.
- ^ Christe, K. O.; Wilson, W. W. (1986). "Synthesis and characterization of NF+
4BrF−
4 and NF+
4BrF
4O−
". Inorganic Chemistry. 25 (11): 1904–06. doi:10.1021/ic00231a038. - ^ a b c Murthy, C. Parameshwara (2008). University Chemistry, Tom 1. New Age International. pp. 180–182, 206–208. ISBN 978-8122407426.
- ^ Crawford, M.; Klapötke, T. M. (1999). "The trifluorooxonium cation, OF+
3". Journal of Fluorine Chemistry. 99 (2): 151–156. doi:10.1016/S0022-1139(99)00139-6. - ^ Wiberg, Wiberg & Holleman 2001, p. 435.
- ^ Wiberg, Wiberg & Holleman 2001, p. 436.
- ^ a b Pitzer, Kenneth Sanborn, ed. (1993). Molecular Structure and Statistical Thermodynamics: Selected Papers of Kenneth S. Pitzer. Vol. 1. World Scientific. p. 111. ISBN 9810214391.
- ^ Gmelin, Leopold. Gmelin Handbook of inorganic chemistry: At--Astatine (8th ed.). Springer-Verlag. p. 224. ISBN 9783540935162.
- ^ Greenwood & Earnshaw 1998, pp. 828–830.
- ^ Patnaik, Pradyot (2007). A Comprehensive Guide to the Hazardous Properties of Chemical Substances. Hoboken, New Jersey: John Wiley & Sons. pp. 478–479. ISBN 9780471714583.
- ^ a b Olah, George A. (2005). "Crossing conventional boundaries in half a century of research". Journal of Organic Chemistry. 70 (7): 2413–29. doi:10.1021/jo040285o. PMID 15787527.
- ^ "The Nobel Prize in Chemistry 1994". The Nobel Foundation. Retrieved 22 December 2008.
- ^ Wiberg, Wiberg & Holleman 2001, pp. 392–393.
- ^ Chemical and Engineering News as cited by Michael Barnes. "Neil Bartlett, emeritus professor of chemistry, dies at 75". University of California Newsroom. Retrieved 24 December 2011.
- ^ Wiberg, Wiberg & Holleman 2001, p. 438.
- ^ Wiberg, Wiberg & Holleman 2001, p. 400.
- ^ Grosse, A. V.; Kirshenbaum, A. D.; Streng, A. G.; Streng, L. V. (1963). "Krypton Tetrafluoride: Preparation and Some Properties". Science. 139 (3559): 1047–1048. Bibcode:1963Sci...139.1047G. doi:10.1126/science.139.3559.1047. PMID 17812982.
- ^ a b Dixon, D. A.; Wang, T. H.; Grant, D. J.; Peterson, K. A.; Christe, K. O.; Schrobilgen, G. J. (2007). "Heats of Formation of Krypton Fluorides and Stability Predictions for KrF4 and KrF6 from High Level Electronic Structure Calculations". Inorganic Chemistry. 46 (23): 10016–10021. doi:10.1021/ic701313h. PMID 17941630.
- ^ Henderson, W. (2000). Main Group Elements. Royal Society of Chemistry. p. 151. ISBN 0854046178.
- ^ Xu, Ruren; Pang, Wenqin; Huo, Qisheng (2011). Main Inorganic Synthetic Chemistry. p. 54. ISBN 9780444535993.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Basting, D.; Marowsky, G. (2005). "Historical Review of the Excimer Laser Development". Excimer Laser Technology. Springer. pp. 8–20. ISBN 978-3-540-20056-7.
- ^ Lewars 2008, pp. 70–71.
- ^ Chaban, Galina M.; Lundell, Jan; Gerber, R. Benny (2001). "Lifetime and decomposition pathways of a chemically bound helium compound". The Journal of Chemical Physics. 115 (16): 7341–44. Bibcode:2001JChPh.115.7341C. doi:10.1063/1.1412467.
- ^ Lias, S. G.; Liebman, J. F.; Levin, R. D. (1984). "Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules". Journal of Physical and Chemical Reference Data. 13 (3): 695. Bibcode:1984JPCRD..13..695L. doi:10.1063/1.555719.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lewars 2008, pp. 70–78.
- ^ Steigerwald, F.; Walter, W.; Langhoff, H.; Hammer, W. (1988). "Emission spectrum and formation kinetics of neon fluoride". Zeitschrift für Physik D. 7 (4): 379–382. Bibcode:1988ZPhyD...7..379S. doi:10.1007/BF01439807. S2CID 122514781.
- ^ Han, Young-Kyu; Lee, Yoon Sup (1999). "Structures of RgFn (Rg = Xe, Rn, and element 118. n = 2, 4.) Calculated by two-component spin-orbit methods. A spin-orbit induced isomer of (118)F4" (PDF). Journal of Physical Chemistry A. 103 (8): 1104–08. doi:10.1021/jp983665k.
- ^ Barber, Robert C.; Karol, Paul J.; Nakahara, Hiromichi; Vardaci, Emanuele; Vogt, Erich W. (2011). "Discovery of the elements with atomic numbers greater than or equal to 113 (IUPAC Technical Report)". Pure and Applied Chemistry. 83 (7): 1. doi:10.1351/PAC-REP-10-05-01. S2CID 98065999.
- ^ Wang, Xuefang; Andrews, Lester; Riedel, Sebastian; Kaupp, Martin (2007). "Mercury is a transition metal: The first experimental evidence for HgF4". Angewandte Chemie. 119 (44): 8523–27. doi:10.1002/ange.200703710.
- ^ Jensen, William B. (2008). "Is mercury now a transition element?". Journal of Chemical Education. 85 (9): 1182–1183. Bibcode:2008JChEd..85.1182J. doi:10.1021/ed085p1182.
- ^ a b Wiberg, Wiberg & Holleman 2001, p. 398.
- ^ Haire, Richard G. (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Springer Science+Business Media. p. 1723. ISBN 1-4020-3555-1.
{{cite book}}: CS1 maint: ref duplicates default (link) - ^ Polysciences, Inc. (2000). "Technical Data Sheet 320 Ruthenium Tetroxide" (PDF). p. 1.
- ^ Bayerische Julius-Maximilians-Universität Würzburg 2006, p. 34.
- ^ O'Hagan, D. (2008). "Understanding organofluorine chemistry. An introduction to the C–F bond". Chemical Society Reviews. 37 (2): 308–19. doi:10.1039/b711844a. PMID 18197347.
- ^ a b Cite error: The named reference
Jstgwas invoked but never defined (see the help page). - ^ a b Sukornick, B. (1989). "Potentially acceptable substitutes for the chlorofluorocarbons". International Journal of Thermophysics. 10 (3): 553–61. Bibcode:1989IJT....10..553S. doi:10.1007/BF00507978. S2CID 128953494.
- ^ McMurry, John (2009). Organic Chemistry: With Biological Applications. Mary Finch. p. 618. ISBN 978-0-495-39145-6. Retrieved 9 April 2011.
- ^ University of California, Berkeley. "Research into Gecko Adhesion". Archived from the original on 14 October 2007. Retrieved 29 April 2011.
Cite error: There are <ref group=note> tags on this page, but the references will not show without a {{reflist|group=note}} template (see the help page).