Wikipedia:Reference desk/Archives/Science/2018 December 31
| Science desk | ||
|---|---|---|
| < December 30 | << Nov | December | Jan >> | January 1 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
December 31
[edit]Weapons of REAL mass destruction
[edit]Is it possible to cause the Baltic Sea and/or the Caspian Sea to explode? If so, about how big of an explosion (in megatons) could happen in this scenario, and how much damage on Russian soil could it cause? Is it possible to do the same thing with permafrost? 2601:646:8A00:A0B3:F484:63E7:CDC0:1004 (talk) 10:53, 31 December 2018 (UTC)
- Well, the Schuylkill River and the Cuyahoga River have caught fire at various times, so anything's possible. ←Baseball Bugs What's up, Doc? carrots→ 11:17, 31 December 2018 (UTC)
- Hydrogen sulfide produced from the microbial breakdown of organic matter may be delivered in small amounts from sewers to the seas mentioned but it is not explosive, merely smelly. DroneB (talk) 14:15, 31 December 2018 (UTC)
- I think the OP might be thinking of the Lake Nyos disaster, which depended on carbon dioxide release - once an area started bubbling up, it had a positive feedback until a large amount of CO2 headed toward the village. Laboratory mice are euthanized the same way (also occasional hip kids who try dry ice in a hot tub). Hydrogen sulfide can be emitted during such events; I remember it, or other gases (I see our article mentions methane clathrates) was an explanation given for the Permian extinction but there were a lot of horses in that race and I haven't looked at the standings in some time. I remember long back reading the Black Sea has a high CO2 content deep down, so the idea using a shipload of dry ice to gas Turkey was an old fave, but I wouldn't bet on it. Most of these events are blamed on volcanism that has been priming waters to the point of a catastrophic turnover for some time, which is to say, they are repeated events; if you don't see that a body of water has done it before, I doubt puny humans can easily make it happen a first time. Wnt (talk) 15:18, 31 December 2018 (UTC)
- Actually, I was thinking of the Status-6 first-strike nuclear torpedo which the Russians have developed (along with other ideas of theirs, such as nuking Yellowstone to make it erupt, or blowing up Iceland to disrupt the Gulf Stream), and looking at ways we might do the same things to them in retaliation -- my idea was to explode a nuclear weapon (say, a B83 fitted with a hydrostatic fuze) just below the halocline in the Gotland Deep and/or in the Caspian Sea in order to disrupt the halocline and cause massive outgassing of hydrogen sulfide (hopefully creating an explosive fuel-air mixture which the fireball would promptly detonate, hopefully causing a self-sustaining chain reaction which would cause most of the dissolved hydrogen sulfide to explode and create a veritable (and radioactive) tsunami heading toward St. Petersburg and Astrakhan and hopefully far inland). 2601:646:8A00:A0B3:F484:63E7:CDC0:1004 (talk) 02:55, 1 January 2019 (UTC)
- How nice. Please find a forum somewhere else on the Internet for such ghoulish speculation. Acroterion (talk) 03:02, 1 January 2019 (UTC)
- Please cite Wikipedia policy which prohibits discussion of this subject on here, or else retract your comment. 2601:646:8A00:A0B3:F484:63E7:CDC0:1004 (talk) 03:10, 1 January 2019 (UTC)
- "The reference desk is not a chatroom, nor is it a soapbox for promoting individual opinions" and "The reference desk is not a place to debate controversial subjects." To extend, the refdesk isn't a forum for gleeful spitballing about "hopefully" creating radioactive tsunamis to destroy Russia in speculative forum-style posts. Your initial question has been answered, as far as I can tell, take the fictional speculation elsewhere. Acroterion (talk) 03:32, 1 January 2019 (UTC)
- In that case, I have 2 specific questions here which have to do with science: (1) In the scenario I outlined above, will the gas (hydrogen sulfide or methane) detonate? (2) If so, will this detonation cause a self-sustaining and self-propagating chain reaction? 2601:646:8A00:A0B3:F484:63E7:CDC0:1004 (talk) 05:29, 1 January 2019 (UTC)
- There are some things I don't know about this ... to be honest, I never even realized the Baltic Sea was brackish, even what our article calls "borderline freshwater", until now. (Except for the salty deep region described [1]) But what I do know is that H2S and H4C will not "detonate", because detonation implies that all the ingredients for a chemical reaction are present. Whether they could or would burn is another question, which I don't know the answer to. Methane, of course, is more dangerous if it doesn't burn, hence natural gas flare stacks. Creating a tsunami is a dicey proposition, since colossal energies from earthquakes often fail to produce one even when they are feared, and no nuclear weapon I know of can register 8 or 9 on the Richter scale. Supervillains might take heart that the source of a catastrophic tsunami might be predicted and manipulated, but if you look at a map of a feared feature [2], it's not like it has a "just push here" sign; who knows if even a lot of nukes would do it? Additionally, a radioactive source for a tsunami does not produce a radioactive tsunami, since a gravity wave does not literally mean that water propagates from source to destination, only that its displacement propagates. Wnt (talk) 16:16, 1 January 2019 (UTC)
- So the answer is "probably not"? 2601:646:8A00:A0B3:F484:63E7:CDC0:1004 (talk) 01:37, 2 January 2019 (UTC)
- There are some things I don't know about this ... to be honest, I never even realized the Baltic Sea was brackish, even what our article calls "borderline freshwater", until now. (Except for the salty deep region described [1]) But what I do know is that H2S and H4C will not "detonate", because detonation implies that all the ingredients for a chemical reaction are present. Whether they could or would burn is another question, which I don't know the answer to. Methane, of course, is more dangerous if it doesn't burn, hence natural gas flare stacks. Creating a tsunami is a dicey proposition, since colossal energies from earthquakes often fail to produce one even when they are feared, and no nuclear weapon I know of can register 8 or 9 on the Richter scale. Supervillains might take heart that the source of a catastrophic tsunami might be predicted and manipulated, but if you look at a map of a feared feature [2], it's not like it has a "just push here" sign; who knows if even a lot of nukes would do it? Additionally, a radioactive source for a tsunami does not produce a radioactive tsunami, since a gravity wave does not literally mean that water propagates from source to destination, only that its displacement propagates. Wnt (talk) 16:16, 1 January 2019 (UTC)
- In that case, I have 2 specific questions here which have to do with science: (1) In the scenario I outlined above, will the gas (hydrogen sulfide or methane) detonate? (2) If so, will this detonation cause a self-sustaining and self-propagating chain reaction? 2601:646:8A00:A0B3:F484:63E7:CDC0:1004 (talk) 05:29, 1 January 2019 (UTC)
- "The reference desk is not a chatroom, nor is it a soapbox for promoting individual opinions" and "The reference desk is not a place to debate controversial subjects." To extend, the refdesk isn't a forum for gleeful spitballing about "hopefully" creating radioactive tsunamis to destroy Russia in speculative forum-style posts. Your initial question has been answered, as far as I can tell, take the fictional speculation elsewhere. Acroterion (talk) 03:32, 1 January 2019 (UTC)
- Please cite Wikipedia policy which prohibits discussion of this subject on here, or else retract your comment. 2601:646:8A00:A0B3:F484:63E7:CDC0:1004 (talk) 03:10, 1 January 2019 (UTC)
- How nice. Please find a forum somewhere else on the Internet for such ghoulish speculation. Acroterion (talk) 03:02, 1 January 2019 (UTC)
- Actually, I was thinking of the Status-6 first-strike nuclear torpedo which the Russians have developed (along with other ideas of theirs, such as nuking Yellowstone to make it erupt, or blowing up Iceland to disrupt the Gulf Stream), and looking at ways we might do the same things to them in retaliation -- my idea was to explode a nuclear weapon (say, a B83 fitted with a hydrostatic fuze) just below the halocline in the Gotland Deep and/or in the Caspian Sea in order to disrupt the halocline and cause massive outgassing of hydrogen sulfide (hopefully creating an explosive fuel-air mixture which the fireball would promptly detonate, hopefully causing a self-sustaining chain reaction which would cause most of the dissolved hydrogen sulfide to explode and create a veritable (and radioactive) tsunami heading toward St. Petersburg and Astrakhan and hopefully far inland). 2601:646:8A00:A0B3:F484:63E7:CDC0:1004 (talk) 02:55, 1 January 2019 (UTC)
- The simplest way is to let an asteroid impact the target. NASA is investigating methods to avert asteroid strikes by changing the course of potential impactors, but the same methods can be used to steer an asteroid to hit a target on Earth. The asteroid that will be made to hit Earth doesn't have to be targeted directly, one may seek out a smaller asteroid whose course can be changed to hit a larger asteroid that will in turn hit the Earth. For the purpose of avoiding collisions with the Earth, I've shown here that this method to target smaller asteroids to hit the larger asteroids of interests, can be more practical than trying to deflect the larger asteroid directly. Count Iblis (talk) 19:27, 2 January 2019 (UTC)
Is superheated water a unique phase of water?
[edit]If the phase transition to superheated water only occurred when water temperature exceeded 100°C, then water would never superheat. Boiling would PREVENT superheating. Therefore, water must completely convert into the phase that can superheat BEFORE water can superheat.
Certain transitions between phases do not necessarily require latent heat. The phase transition from water with a 100°C boiling point to water that can superheat occurs when pure water is not in a scratched container and is vibration free. Conversely, vibration or a scratched container can convert the phase that can superheat into the phase with a 100° C boiling point.
Disturbing the superheated water triggered conversion to the phase with the 100°C boiling point. The vapor is 100°C because the phase with the 100°C boiling point is vaporizing.
The phase that can superheat is not boiling. If superheated water itself indeed boiled, its vapor temperature should be the same as its water temperature and boiling should occur at the rate external heat is added.
The phase that can superheat has a lower melting point than the phase that boils at 100°C. Likewise, there are 18 known solid crystalline phases of water, each with their own phase transition temperatures and pressures.
- “Extremely pure, supercooled water stays liquid below 0°C and remains so until applied vibrations or condensing seed doping initiates crystallization centers. This is a common situation for the droplets of atmospheric clouds.” — Preceding unsigned comment added by Vze2wgsm1 (talk • contribs) 11:30, 31 December 2018 (UTC)
Vze2wgsm1 (talk) 11:32, 31 December 2018 (UTC)
- Superheated water isn't a different phase but there is evidence that liquid water is really strange in that there are two phases of liquid water with a crossover between 40°C and 60°C. Dmcq (talk) 13:23, 31 December 2018 (UTC)
- @Dmcq: do you want to say more about that? I don't know what you mean. --Trovatore (talk) 01:44, 1 January 2019 (UTC)
- [3], in particular there are some studies linking this to the temperature at which proteins denature if you look at the papers citing it. Dmcq (talk) 02:04, 1 January 2019 (UTC)
- @Dmcq: do you want to say more about that? I don't know what you mean. --Trovatore (talk) 01:44, 1 January 2019 (UTC)
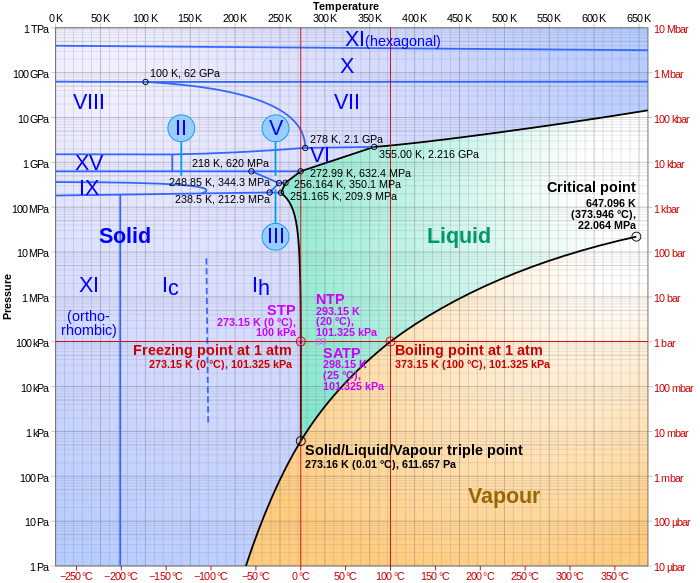
- Boiling does prevent superheating. When you heat pure water past its proper boiling point on that phase diagram (100 C at 1 atm), then logically two things can happen. Either it turns into a gas, in which case you say it boils, or it stays liquid, in which case you say it superheated. The key fact that matters here is that boiling doesn't happen on a single-molecule level; it requires nucleation. Think of an angry mob facing off against a line of cops -- there has to be somebody to throw the first stone. If your water isn't very pure, there will be some "troublemakers" around to make sure that happens as soon as it can, but if it is totally homogeneous then you can see some stranger behavior. You can be at a point where a large bubble would get bigger, but no bubble exists to begin with. I recall an intro chem lab where my entire reaction in petroleum ether abruptly took to the air - bumping (chemistry) - leaving reactants/product all over the benchtop and what was almost immediately a dry Erlenmeyer behind. Wnt (talk) 15:26, 31 December 2018 (UTC) Does not apply. Petroleum ether is not a pure compound. Vze2wgsm1 (talk) 02:11, 1 January 2019 (UTC)
For all pure compounds, boiling occurs at temperature and pressure combinations where added thermal energy converts into heat of vaporization, instead of increasing enthalpy. Each of these temperature/pressure combinations are precise.
A requirement for nucleation sites would make boiling points imprecise. Therefore, no pure compound requires nucleation sites to initiate boiling.
Superheated water contains more enthalpy than water that boils at 100 C, without becoming less stable than a vapor molecule. The phase of water that boils at 100 C becomes less stable than a vapor molecule if temperature exceeds 100 C. Therefore, water that can superheat is a different pure chemical compound than water that boils at 100 C.
Note: The boiling point of a pure compound is not necessarily a function of surface tension. Adding surfactants to change water’s surface tension does not necessarily lower water’s boiling point.
- “They found that adding surfactants to the water jet did not change the incipient boiling point.” Vze2wgsm1 (talk) 01:34, 1 January 2019 (UTC)
- I think you're probably getting confused by that paper, 'supercritical' is not the same as 'superheated'. Dmcq (talk) 12:03, 1 January 2019 (UTC)
- In the paper, the author used the word supercritical was with respect to Nusselt numbers, not to the phase of water with the high Nusselt numbers. The water in the supercritical region was (initially) subcooled.
- “Boiling was not present in the supercritical region of flow partly because water entered the channel as a subcooled liquid, and partly because the supercritical flow field had high enough heat transfer coefficients to keep the disk surface below the level of superheat needed to induce boiling.”Vze2wgsm1 (talk) 14:48, 1 January 2019 (UTC)
What are sweet peas?
[edit]This is related to my seeing Popeye (film) for the first time. When I went to Sweet pea I was expecting a plant that we can eat, but the article says we shouldn't eat it. However, I have heard for years about "sweet peas", including a product by that name that was related to a pricing game on The Price Is Right. Nothing in the pea article seems to suggest there is such a thing that we can eat.— Vchimpanzee • talk • contributions • 18:21, 31 December 2018 (UTC)
- Pisum sativum, regular garden peas, vary in their sugar content according to cultivar, ripeness, and processing. The sweeter ones are called sweet peas. Abductive (reasoning) 19:01, 31 December 2018 (UTC)
- Could this be the snow pea? Its scientific name is P. sativum var. saccharatum, i.e. "sugared" or something like that; in German it is called de:Zuckererbse, i.e. "sugar pea". --Wrongfilter (talk) 19:34, 31 December 2018 (UTC)
- I don't know. Snap pea comes closer but I don't see anything in the article calling it "sweet pea".— Vchimpanzee • talk • contributions • 20:13, 31 December 2018 (UTC)
- It's called sugar snap pea per our article or simply sugar pea [4] [5] [6] [7] en:wiktionary:sugar pea sometimes. I guess it's possible some people may call it sweet pea, but I doubt it's very common. Nil Einne (talk) 22:42, 31 December 2018 (UTC)
- Our article on the Popeye character Swee'Pea states that the name derives from sweet pea (Lathyrus odoratus). The name "sweet pea" in horticulture normally refers to this plant, rather than to a sweet-tasting edible pea. My guess is that the "sweet" part of the common name is referring to the scent of the flowers, not the taste of any fruit (peas). PaleCloudedWhite (talk) 23:07, 31 December 2018 (UTC)
- As with the flowering plant called Sweet William. ←Baseball Bugs What's up, Doc? carrots→ 03:50, 1 January 2019 (UTC)
- To confirm PaleCloudedWhite's guess, the OED says that the Sweet Pea was 'formerly called sweet-scented pea'. AndrewWTaylor (talk) 10:23, 1 January 2019 (UTC)
- Our article on the Popeye character Swee'Pea states that the name derives from sweet pea (Lathyrus odoratus). The name "sweet pea" in horticulture normally refers to this plant, rather than to a sweet-tasting edible pea. My guess is that the "sweet" part of the common name is referring to the scent of the flowers, not the taste of any fruit (peas). PaleCloudedWhite (talk) 23:07, 31 December 2018 (UTC)
- It's called sugar snap pea per our article or simply sugar pea [4] [5] [6] [7] en:wiktionary:sugar pea sometimes. I guess it's possible some people may call it sweet pea, but I doubt it's very common. Nil Einne (talk) 22:42, 31 December 2018 (UTC)
- I don't know. Snap pea comes closer but I don't see anything in the article calling it "sweet pea".— Vchimpanzee • talk • contributions • 20:13, 31 December 2018 (UTC)
- Could this be the snow pea? Its scientific name is P. sativum var. saccharatum, i.e. "sugared" or something like that; in German it is called de:Zuckererbse, i.e. "sugar pea". --Wrongfilter (talk) 19:34, 31 December 2018 (UTC)
What CO2 percent is needed to boil the ocean?
[edit]If you added it in a climatological instant like a year. How long would it take to start and finish boiling? How long after that before thermal equilibrium? Would any carbonate rocks survive? How hot would Challenger Deep and Mount Everest get? Sagittarian Milky Way (talk) 22:48, 31 December 2018 (UTC)
- I'm assuming you mean, 'what CO2 percent in the atmosphere...'? PaleCloudedWhite (talk) 23:19, 31 December 2018 (UTC)
- "Numerical climate models as well as carbon isotope measurements from preserved Ordovician soils suggest that atmospheric levels of carbon dioxide during the period were 14–16 times higher than today... How continental glaciation could have formed when carbon dioxide levels were so high has been a paradox."[8] If you look at the various theories, you will find a vigorous debate about glaciers forming vs. glaciers melting during this high CO2 period, but the debate is about temperatures slightly above freezing vs, temperatures slightly below freezing, never about temperatures near boiling.
- As our article on Runaway climate change says, "an extreme moist greenhouse might cause an instability with water vapour preventing radiation to space of all absorbed solar energy, resulting in very high surface temperature and evaporation of the ocean. However, simulations indicate that no plausible human-made greenhouse gas (GHG) forcing can cause an instability and baked-crust runaway greenhouse effect." --Guy Macon (talk) 00:38, 1 January 2019 (UTC)
- The following is a lot more speculative, but our article on Snowball Earth says "The carbon dioxide levels necessary to unfreeze Earth have been estimated as being 350 times what they are today, about 13% of the atmosphere."
- Also our article on Carbon dioxide in Earth's atmosphere says "Concentrations of CO2 in the atmosphere were as high as 4,000 parts per million (ppm) during the Cambrian period about 500 million years ago to as low as 180 ppm during the Quaternary glaciation of the last two million years... Global annual mean CO2 concentration has increased by more than 45% since the start of the Industrial Revolution, from 280 ppm during the 10,000 years up to the mid-18th century to 410 ppm as of mid-2018." The earth has never experienced boiling oceans since the time during and shortly after the surface was molten lava, but we have seen periods of little or no glaciation, alternating with ice ages. --Guy Macon (talk) 00:51, 1 January 2019 (UTC)
- Note the relevant article/term is runaway greenhouse effect or runaway climate change. It seems to be relatively out of favor as a possibility lately, but some do say it could happen. I've been waiting for old artifacts from Venusian exploration missions to turn up and provide some insight on this... really was holding out some hope for the Dawn mission... Some of the NASA maps I've seen lately put Earth right at the inner edge of the Sun's habitable zone, FWIW. Note that the question is difficult and would require simulation because to get to the boiling of oceans implies not just a few degrees of heating but a "runaway" proper, i.e. that the increased water vapor acts as a greenhouse gas more than the increased clouds act to cool the planet. At the same time, the remarkable CO2 levels quoted from long ago have to be taken in context of the faint young Sun paradox; it isn't clear nearly that much would be needed now. Wnt (talk) 05:27, 1 January 2019 (UTC)
- Very roughly, a runaway moist greenhouse might start about 47 C in the global mean temperature according to runaway greenhouse effect. That's about 33 C warmer than today. Assuming a climate sensitivity of ~3 C/doubling of CO2, that would imply something like 2000 times as much CO2 as today. Which translates to adding enough CO2 that roughly 1/2 of the atmosphere would be CO2 (assuming other constituents stay the same). At that level, all macroscopic oxygen-dependent life would suffocate before they had a chance to boil. Dragons flight (talk) 11:21, 1 January 2019 (UTC)
- If tenths of a bar of CO2 was suddenly added would enough of the locked-up methane be released before its atmosphere residence time to matter? Sagittarian Milky Way (talk) 14:24, 1 January 2019 (UTC)
- @Dragons flight: That article describes an effect per "doubling", but as far as I see they only look at the one data point of double some baseline. I don't think they're making a claim for a logarithmic effect, are they? At least, I don't see why the effect would be logarithmic, though I don't know it wouldn't be either. Given the degree of uncertainty for even one data point, in any case, I would be skeptical that the curve of many can be predicted beyond the parameters of existing simulations. Wnt (talk) 16:04, 1 January 2019 (UTC)
- The radiative forcing of CO2 is approximately logarithmic. For more complete expressions, I would refer you to Table 6.2 of this IPCC chapter [9]. The logarithmic response arises (primarily) because the radiation absorption on the wings of the CO2 absorption bands evolve approximately exponentially with distance from the center on the band. The climate response (e.g. 3C) to a given radiative forcing is quite uncertain (due largely to various feedbacks), but the radiative forcing part as a function of CO2 actually isn't very uncertain (at least not at normal concentrations). I would however assume that there is a very large uncertainty with extrapolating to thousands of times modern CO2 levels, but then I also assume that SMW isn't really in need of a very precise answer. Dragons flight (talk) 18:02, 1 January 2019 (UTC)
- Thanks! That chapter looks like the Real Deal for understanding this stuff, and quite a lot of it. Wnt (talk) 20:53, 1 January 2019 (UTC)
